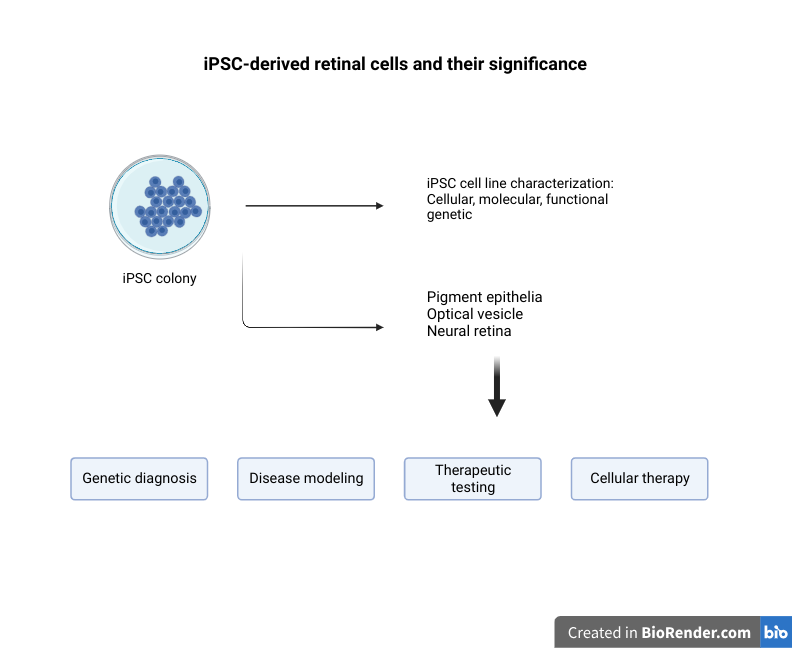
Character And Morphology Of Spheroids
Spheroid cell culture is a type of scaffold-free 3D cell culture method. Spheroids are formed by the self-assembly of cells. Generally, spheroid formation takes place in 3 steps (1-3).
1. Formation of loosely packed cell aggregates via integrin-ECM binding
2. Expression and accumulation of cadherins
3. Formation of tightly packed spheroids through cadherin-cadherin interaction.
Spheroids formed in this manner include comprehensive cell-cell and cell-ECM connections that mimic in vivo-like cellular interactions. When compared to traditional 2D cell culture, spheroids show improved cell viability, proliferation and differentiation and secretion of proteins (1-3). For example, primary hepatocyte-derived spheroids display in vivo-like cellular architecture for over 4 weeks, together with intact hepatic liver specific functions (4). Other cell types like mesenchymal stem cells form spheroids whose secretome has shown to have a different composition compared to the one observed in 2D cultures, with a higher concentration of anti-inflammatory factors. (5,6). Spheroid vitality and consistency are determined by morphological features such as spheroid size, shape uniformity and cell density.
The Size
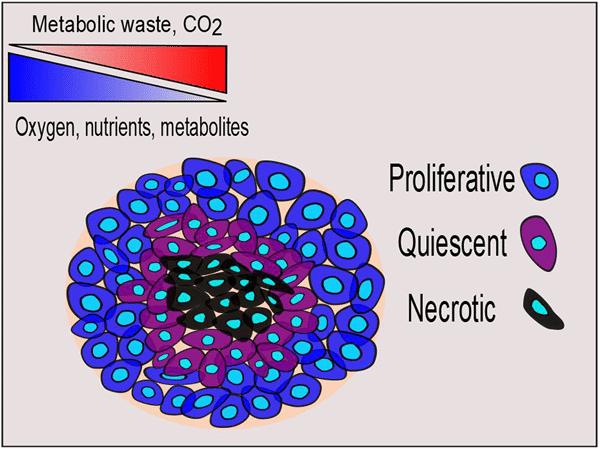
Different properties of spheroids are primarily determined by their size. Spheroids often show a three-layered structure, with a proliferative, a quiescent and a necrotic layer. The proliferative layer is the outermost layer and contains metabolically active cells. The middle layer is the quiescent layer in which the metabolic activity is minimal, and cells are found in a quiescent stage when exposed to nutrients. In the core of the spheroid, necrotic cells can be found due to the lack of nutrients and the accumulation of waste (7). Under normal culturing conditions, the diameter of a spheroid needs to be maintained in a range between 100 and150 μm to achieve uniform cell viability within the whole 3D structure. Continuous oxygen supply with microfluidic techniques would help maintain cell proliferation, viability, and cellular functions in spheroids with a diameter of 600 μm (8).
The mass of some solid tumors is characterized by a necrotic core. Tumor spheroids can be generated in such a way that the size of the spheroid is intentionally increased to form a hypoxic inner center that is similar to what is found within the tumor microenvironment. Thus, the optimal size of spheroids depends on the type of cell, the research purposes, and their applications.
The shape
The shape of a spheroid also influences its characteristics. The circularity of spheroids is indicative of oxygen diffusion gradients. More circular spheroids are indicative of even diffusion gradients, low growth rates and higher drug response in drug screenings. In some instances, spheroids can also be generated in an elliptical shape for varied purposes (9,10). However, the spherical shape is still the preferred option to better mimic in vivo-like metabolism and ensure reproducibility across different applications.
Cell density
Cell density is an important factor to consider especially when spheroids are formed by centrifugal force. In this case, the cell density will in turn determine the quality of cell-ECM and cell-cell interaction (11).
While spheroids are generally formed in the same self assembly feature, their size, shape and cell density will determine its properties and characteristics and influence its purpose and applications.
References
1. Lin, R.Z., Chang, H.Y.: Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 3, 1172–1184 (2008).
2. Cui, X., Hartanto, Y., Zhang, H.: Advances in multicellular spheroids formation. J. R Soc. Interface 14, 20160877 (2017).
3. Kim, S.J., Kim, E.M., Yamamoto, M., Park, H., Shin, H.: Engineering Multi-Cellular Spheroids for Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 9, e2000608 (2020).
4. Tostoes, R.M., Leite, S.B., Serra, M., Jensen, J., Bjorquist, P., Carrondo, M.J., Brito, C., Alves, P.M.: Human liver cell spheroids in extended perfusion bioreactor culture for repeated-dose drug testing. Hepatology 55, 1227–1236 (2012).
5. Gionet-Gonzales, M.A., Leach, J.K.: Engineering principles for guiding spheroid function in the regeneration of bone, cartilage, and skin. Biomed. Mater. 13, 034109 (2018).
6. Bartosh, T.J., Ylostalo, J.H., Mohammadipoor, A., Bazhanov, N., Coble, K., Claypool, K., Lee, R.H., Choi, H., Prockop, D.J.: Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their anti-inflammatory properties. Proc. Natl. Acad. Sci. U S A 107, 13724–13729 (2010).
7. Cui, X., Hartanto, Y., Zhang, H.: Advances in multicellular spheroids formation. J. R Soc. Interface 14, 20160877 (2017).
8. Anada, T., Fukuda, J., Sai, Y., Suzuki, O.: An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials 33, 8430–8441 (2012).
9. Raghavan, S., Mehta, P., Horst, E.N., Ward, M.R., Rowley, K.R., Mehta, G.: Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity. Oncotarget 7(13), 16948 (2016).
10. Dean, D.M., Napolitano, A.P., Youssef, J., Morgan, J.R.: Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB J 21, 4005–4012 (2007).
11. De Lora, J.A., Velasquez, J.L., Carroll, N.J., Freyer, J.P., Shreve, A.P.: Centrifugal generation of droplet-based 3D cell cultures. SLAS Technol. 25, 436–445 (2020).
12. Acland, Mitchell & Mittal, Parul & Lokman, Noor & Klingler-Hoffmann, Manuela & Oehler, Martin & Hoffmann, Peter. Mass Spectrometry Analyses of Multicellular Tumor Spheroids. PROTEOMICS – Clinical Applications. 12. 1700124 (2017).