Using Biopolymers And Micromachining Technology In Generating Multicellular Spheroids
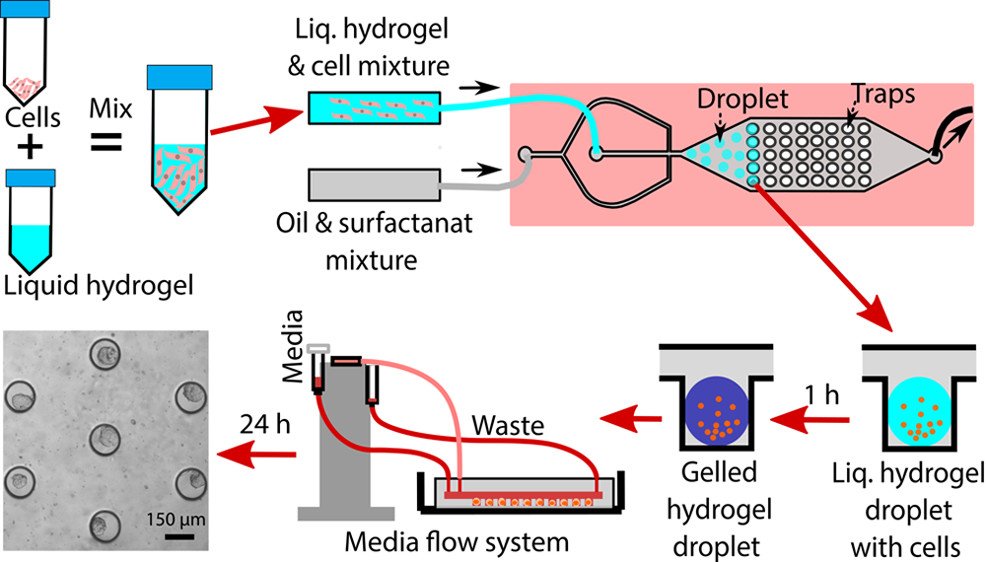
Multicellular spheroids (MCSs) are formed through spontaneous aggregation of cells in the presence of adhesion molecules known as cadherins (1). Decades of research have proved MCSs to be an efficient 3D cell culture model for a wide array of research. Traditional methods employed to generate MCS include liquid overlay, hanging drop method, rotating flask, and using external forces to promote cell aggregation.
These methods rely on the ability of cells to spontaneously aggregate to form multicellular spheroids. However, the above-mentioned methods lack efficiency in promoting cell-cell and cell-extracellular matrix (ECM) interactions. This is primarily because of the inability to recreate the complex cellular microenvironment needed for cell growth. Additionally, traditional methods can influence cell aggregation/proliferation by affecting other parameters such as rigidity, hydrophilicity/hydrophobicity, electric charge and other forces such as gravity, centripetal force, centrifugal force magnetic force and shear force (2-3).
One of the major limitations of the hanging drop method is the rapid use of nutrients and oxygen in the culture environment along with the increase production of metabolic waste as well as osmotic pressure which hamper the generation of spheroids and their subsequent analysis. Other traditional methods such as rotating flasks are effective at generating spheroids for high throughput purposes, but can often lead to cell damage caused by the excessive shear stress. Methods using static cultures do not permit continuous perfusion of nutrients and oxygen and can often become costly due to the use of large amounts of culture medium (4).
Biocompatible hydrogels
In an attempt to overcome the limitations found in traditional methods, perfectly biocompatible hydrogels were introduced, to act as an anchoring agent (scaffold) for ECM formation (5). Methacrylate alginate/multi-arm polyethylene glycol (PEG) double cross-linked hydrogel in micorwell system has been used to generate stem cell spheroids derived from multicellular human adipose tissue.
Hydrogels were found to be ideal in this regard, as the size of the spheroids can be regulated by adjusting the size of the hydrogel microwells . Additionally, the physical and the chemical properties of the hydrogel can be modified locally, to facilitate the formation of a spatially regulated culture system (3).
Naturally formed hydrogels are also used for generating spheroids. These hydrogels are made with biomaterial such as collagen (6), chitosan (7,8), hyaluronic acid (HA) (9) and agar (10). Synthetic hydrogels commonly used include PDMS, PEG, and acrylic acid (4).
Micromachining technology
The term micromachining generally refers to techniques that form topographical features on a surface by means of etching or deposition. These techniques enable researchers to define the biochemical characteristics of a substrate, medium composition and the type of cells surrounding the microenvironment of the other cells with micrometer precision. Furthermore, micromachining technology has the advantage of being a fast, low-cost method that can generate spheroids for high throughput cultivation across multiple culture conditions.
Micromachining devices include microfluidic systems that are proven to be an effective and stable platform for MCS formation. Microfluidic systems provide a dynamic cellular environment and allow for continuous perfusion of culture media, while permitting shear force that can facilitate cell growth and function (11,12).
Over the years, both these technologies; i.e. hydrogels and microfluidic devices have become the go-to method for creating MCSs such as HepG2 spheroids, human colon cancer cell spheroids, and normal human fibroblast spheroids (4).
References
1. Lin, R.Z.; Chou, L.F.; Chien, C.C.; Chang, H.Y. Dynamic analysis of hepatoma spheroid formation: Roles of E-cadherin and beta1-integrin. Cell Tissue Res. 2006, 324, 411–422
2. Ivascu, A.; Kubbies, M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J. Biomol. Screen. 2006, 11, 922–932.
3. Zhang, J.; Yun, S.; Du, Y.; Zannettino, A.C.W.; Zhang, H. Fabrication of a Cartilage Patch by Fusing Hydrogel-Derived Cell Aggregates onto Electrospun Film. Tissue Eng. Part A 2020, 26, 863–871.
4. Shen, H.; Cai, S.; Wu, C.; Yang, W.; Yu, H.; Liu, L. Recent Advances in Three-Dimensional Multicellular Spheroid Culture and Future Development. Micromachines 2021, 12, 96
5. Chen, M.C.W.; Gupta, M.; Cheung, K.C. Alginate-based microfluidic system for tumor spheroid formation and anticancer agent screening. Biomed. Microdevices 2010, 12, 647–654.
6. Kim, C.J.; Terado, T.; Tambe, Y.; Mukaisho, K.I.; Sugihara, H.; Kawauchi, A.; Inoue, H. Anti-oncogenic activities of cyclin D1b siRNA on human bladder cancer cells via induction of apoptosis and suppression of cancer cell stemness and invasiveness. Int. J. Oncol. 2018, 52, 231–240
7. Song, K.; Li, L.; Li, W.; Zhu, Y.; Jiao, Z.; Lim, M.; Fang, M.; Shi, F.; Wang, L.; Liu, T. Three-dimensional dynamic fabrication of engineered cartilage based on chitosan/gelatin hybrid hydrogel scaffold in a spinner flask with a special designed steel frame. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 384–392
8. Wang, K.; Kievit, F.M.; Florczyk, S.J.; Stephen, Z.R.; Zhang, M. 3D Porous Chitosan-Alginate Scaffolds as an In Vitro Model for Evaluating Nanoparticle-Mediated Tumor Targeting and Gene Delivery to Prostate Cancer. Biomacromolecules 2015, 16, 3362–3372.
9. Xu, K.; Narayanan, K.; Lee, F.; Bae, K.H.; Gao, S.; Kurisawa, M. Enzyme-mediated hyaluronic acid-tyramine hydrogels for the propagation of human embryonic stem cells in 3D. Acta Biomater. 2015, 24, 159–171.
10. Tang, Y.; Liu, J.; Chen, Y. Agarose multi-wells for tumour spheroid formation and anti-cancer drug test. Microelectron. Eng. 2016, 158, 41–45.
11. Wu, Y.; Zhao, Z.; Guan, Y.; Zhang, Y. Galactosylated reversible hydrogels as scaffold for HepG2 spheroid generation. Acta Biomater. 2014, 10, 1965–1974.
12. Li N, Tourovskaia A, Folch A. Biology on a chip: microfabrication for studying the behavior of cultured cells. Crit Rev Biomed Eng. 2003;3, 5-6.
