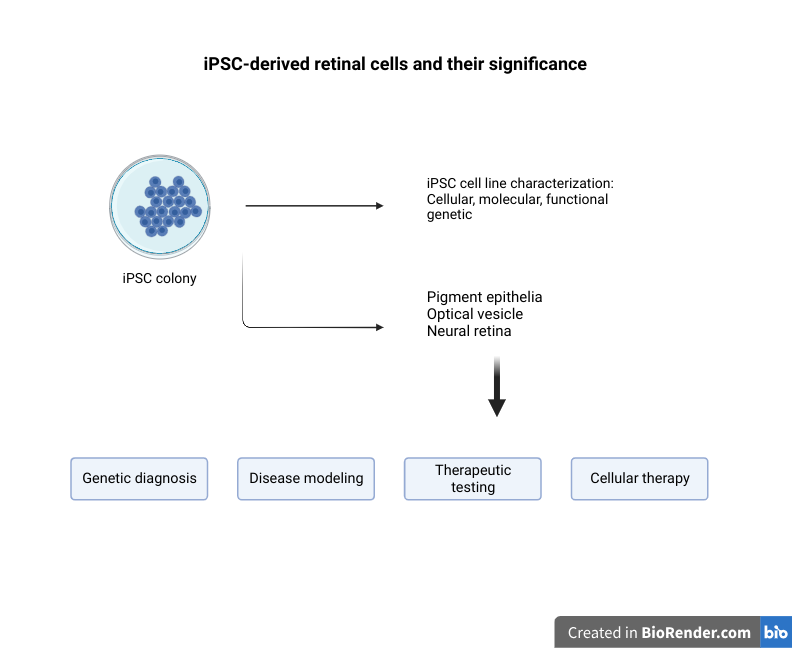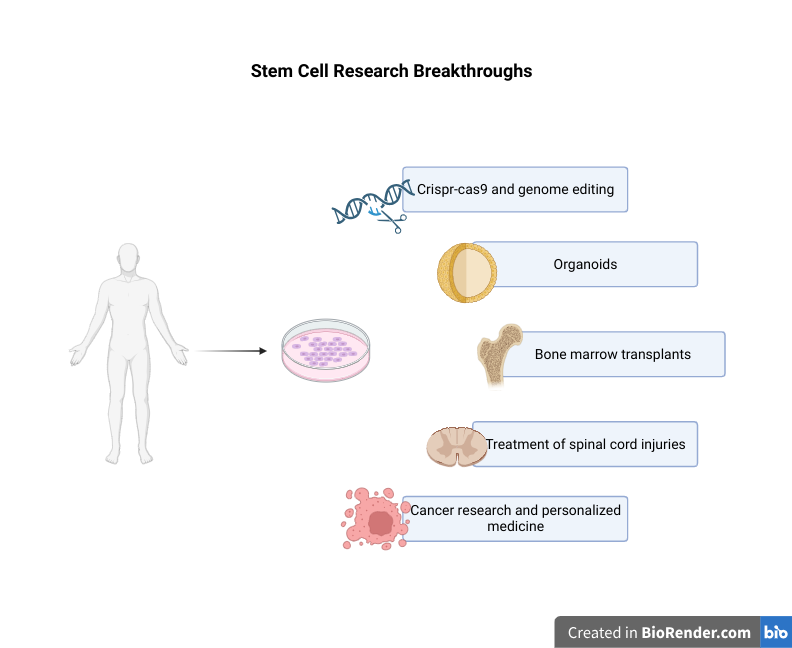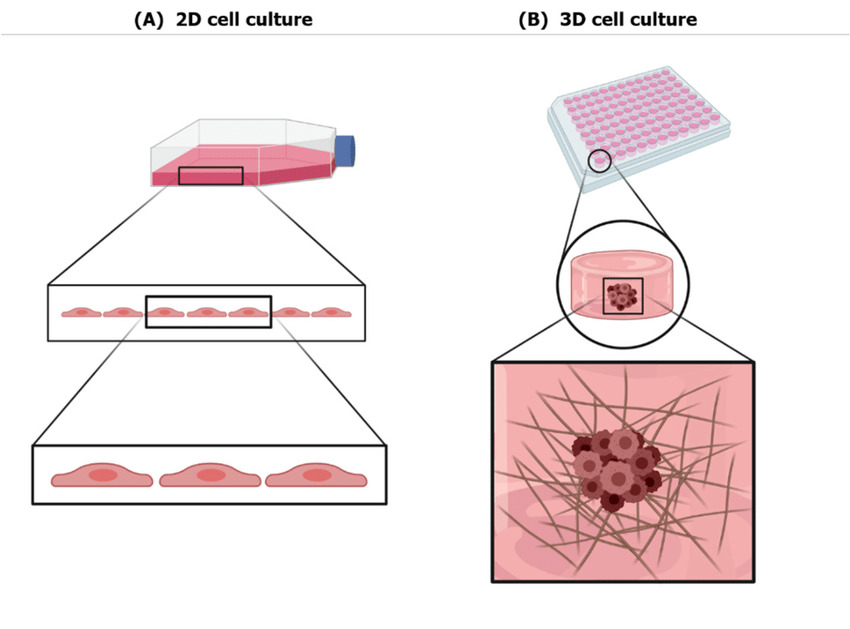
Bridging The Gap Between Three-Dimensional Cultures and Animal Models
Animal models for specific diseases require a pre-existing insight into whether it’s the causative or a genetic condition. They are created by applying harmful conditions to animals or by manipulating the genes involved for a particular disorder respectively, whereas three dimensional models can be generated directly from affected patients without the prior knowledge of the responsible genes involved. Particularly for the multigenic disorders such as inflammatory diseases, if pathology is caused by affected region of epithelium whereas cancer organoids can be directly isolated from the patients (1,2).
Advantages of 3D culture models
3D cell culture models developed from human tissues have several benefits over animal models, in that they organoids provide faster and more robust outcomes, are more readily accessible and provide both a more accurate and a comprehensive representation of human tissues, when compared with traditional animal models. Mouse models are frequently used to explore and study human biology and study diseases, owing to its similarities to human systems. However, generation of transgenic mouse models to address questions regarding human diseases generally consumes more than a year, even with the technology of CRISPR- Cas 9 (Clustered Regularly Interspaced Short Palindromic Repeats) mediated genome editing technology. (3)
Human stem cell derived 3D cultures is widely expected to bridge the gap between animal models and human, because of the source material for culture is from a human. The length of time required to establish the research platform is also faster for 3D models than for animal models, in that human 3D cultures can be established within a few weeks or months with high success rates, thus supporting the use of patient derived 3D cultures in personalized medicine to provide robust personalized data, including the drug responses and individual mutation profiles (4).
REFERENCES:
1. Howell, K. J. et al. DNA methylation and transcription patterns in intestinal epithelial cells from pediatric patients with inflammatory bowel diseases differentiate disease subtypes and associate with outcome. Gastroenterology 154, 585–598 (2018).
2. Drost, J. & Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 18, 407–418 (2018).
3. Yang, H. et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell 154, 1370–1379 (2013).
4. Lancaster, M. A. & Huch, M. Disease modelling in human organoids. Dis. Model Mech. 12, dmm039347 (2019).