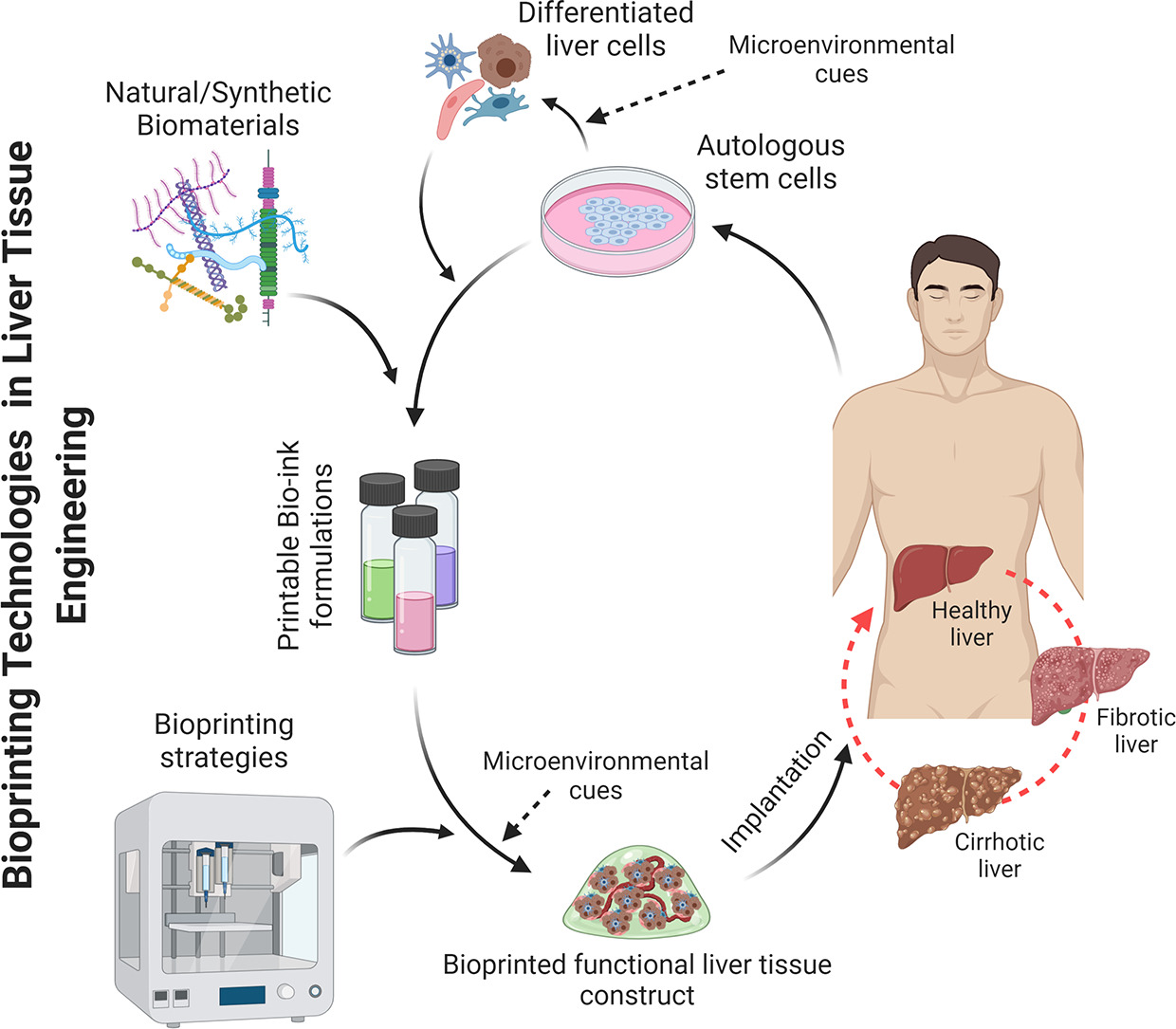
3D printing Hepatic Organoids
The discovery of 3D printing technology accompanied by the development of biocompatible materials such as hydrogels has become a vital tool to advance tissue engineering. 3D bio printing involves cell laden biomaterials called bioinks and layer by layer deposition of cell embedded polymers directed by computer aided software (CAD). Bioprinting is considered a precise and versatile technique that allows to control cell patterning, thus yielding defined cell-cell contacts.
Ideally it is also possible to create in-vivo like extracellular matrix thus paving the way to creating a functional tissue or organ. Hence bioprinting holds great promise in regenerative medicine as well as patient specific therapies, and even in the development of novel devices that can be used for toxicology and drug screening (1,2,5).
Bioprinting the Liver
As of late, there are some viable examples of 3D printed liver cells with enhanced live cell functions. Liver organoids derived from HepaRG and human stellate cells, printed to mimic liver lobules indicated better ALB and CYP3A4 expression compared to traditional monolayer cultures (3). Furthermore, printed hpHep and liver stellate cells maintained urea and ALB production for time period exceeding two weeks while responding to drug treatment, affirming the viability of such cell assays over a long period.
Additionally human 3D printed liver tissues derived from patient specific hepatocytes, and NPCs were also found culture stable for 4 weeks, and other bioprinted liver tissues also indicated stable drug and glucose metabolism, and bile secretion for over three weeks in culture (2,4-5).
Physiologically accurate hepatic structures were also developed by combining iPSC derived hepatic cells together with human umbilical vein endothelial cells and adipose derived SCs to serve as supporting cells. This led to production of in-vivo like hepatic structures with enhanced hepatic specific markers, biotransformation enzymes and albumin and urea production, further attesting to the ability of 3D printing to generate viable, physiologically consistent 3D cultures (2,6).
Overcoming limitations of bioprinting with novel “4D bioprinting”
Despite the technological advances introduced by 3D bioprinitng, it still carries with it some limitations. Namely, limited amount of biomaterials that can be used for printing, low printing speeds, inability to print microstructures and the lack of post printing processes, that are necessary to change scaffolding structures over time, to better mimic the in-vivo cell environment. To overcome the latter barrier, 4D bioprinting has recently been incorporated to increase culture stability over time (I.e the fourth dimension) (7).
In this manner, 4D improves the advantages of 3D by utilising smart materials that are able to reshape themselves in response to different physiological cues such as pH, temperature and light, thus closely resembling the dynamic tissue environment. Together these technologies continue to gain ground as promising techniques for creating physiological relevant, functional liver cell models, to further the developments in regenerative medicine, patient-specific therapies as well as novel devices for drug screens and toxicological research (2,7).
References
1. Ma, J., Wang, Y., and Liu, J. (2018). Bioprinting of 3D tissues/organs combined with microfluidics. RSC Adv. 8, 21712–21727.
2. Serras AS, Rodrigues JS, Cipriano M, Rodrigues AV, Oliveira NG and Miranda JP (2021) A Critical Perspective on 3D Liver Models for Drug Metabolism and Toxicology Studies. Front. Cell Dev. Biol. 9:626805.
3. Grix, T., Ruppelt, A., Thomas, A., Amler, A., Noichl, B., Lauster, R., et al. (2018). Bioprinting Perfusion-Enabled Liver Equivalents for Advanced Organ-on-a-Chip Applications. Genes 9:176.
4. Mazzocchi, A., Devarasetty, M., Huntwork, R., Soker, S., and Skardal, A. (2018). Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 11:015003.
5. Nguyen, D. G., Funk, J., Robbins, J. B., and Crogan-grundy, C. (2016). Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS One 11:e0158674.
6. Ma, C., Zhao, L., Zhou, E. M., Xu, J., Shen, S., and Wang, J. (2016). On-chip construction of liver lobule-like microtissue and its application for adverse drug reaction assay. Anal. Chem. 88, 1719–1727.
7. Gao, B., Yang, Q., Zhao, X., Jin, G., Ma, Y., and Xu, F. (2016). 4D bioprinting for biomedical applications. Trends Biotechnol. 34, 746–756.
8. Agarwal, T., Banerjee, D., Konwarh, R., Esworthy, T., Kumar, Ji., Onesto, V., Das, P., Hoon Lee, B., Wagener, F., Makvandi, P., Mattoli, V., Ghosh, S.K., Maiti, T.K., Zhang, L.G., Ozbolat, I.T. (2021) Recent advances in bioprinting technologies for engineering hepatic tissue, Materials Science and Engineering: C., Volume 123,