Spheroids and three-dimensional stem cell cultures
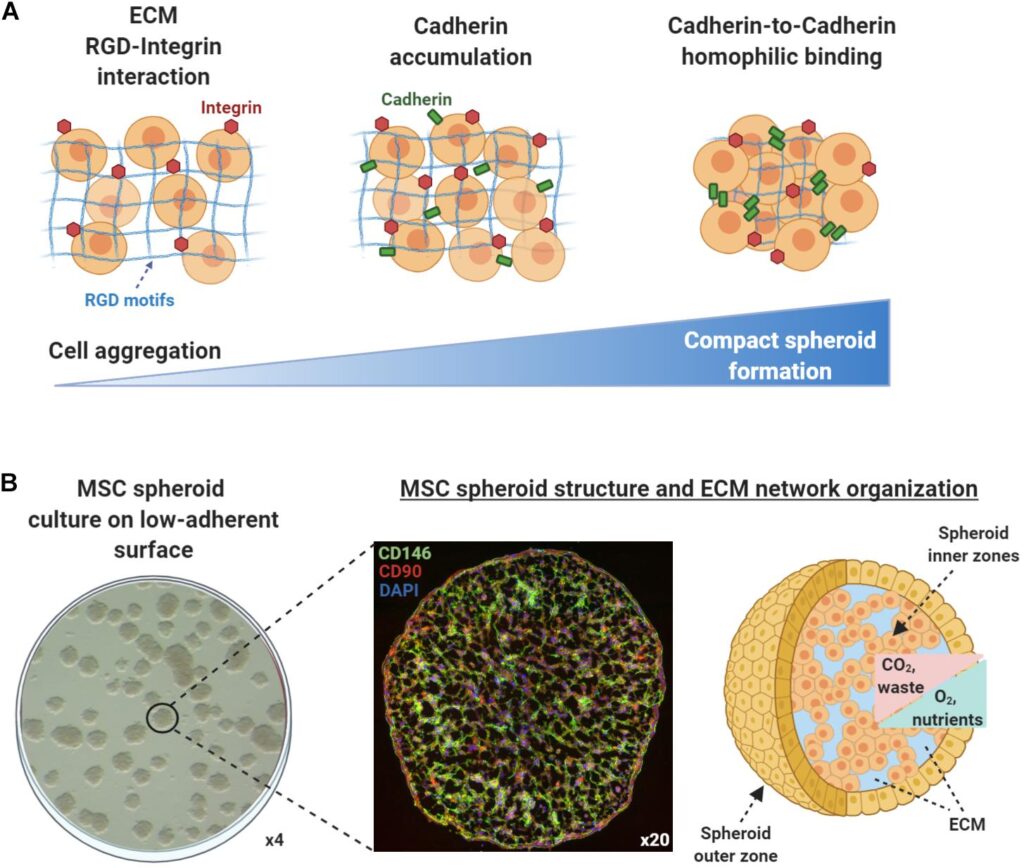
A spheroid culture system provides a similar physiochemical environment to in vivo, by facilitating cell-cell and cell-matrix interaction to overcome the limitations of traditional monolayer cell cultures. Due to the importance of stem cell culture systems in clinical applications, researchers have studied them to optimise the culture conditions and increase efficiency.
In suspension cultures, adjacent cells form aggregates with a spheroidal shape, and they have a vital role in cancer research, drug screening and clinical studies. There are various spheroid culture methods such as hanging drop, gel embedding, magnetic levitation and spinner culture. Various kinds of biomaterials are used as building forms to study spheroids, such as hydrogels, particles, films, and beads, depending on the experimental requirements (1,2).
Stem cells
Stem cells are valuable key players in regenerative medicine with clinical and research applications. The primary characteristic of stem cells is their stemness, which is the ability to generate daughter cells that are identical to their mother (self-renewal). Their multipotency allows the differentiation into new cell types with limited proliferation potential. Stemness enables stem cells to have potential for application in numerous biological therapeutic tools such as high-throughput pharmacology, drug screening and tissue engineering (3,4).
Two-dimensional versus Three-dimensional cell cultures
Cells require biological signals in the microenvironment of cellular niches. These signals encourage proliferation and enhance cellular viability. In general, traditional two-dimensional cell culture systems, wherein the cells grow as a monolayer, face some limitations in reproducing the in vivo microenvironment. On the contrary, three-dimensional cell culture systems can reconstitute conditions like the ones observed in vivo and can build cell-cell and cell-matrix interaction networks (5,6).
REFERENCES:
1. Na-Eun Ryu , 1Department of Integrative Engineering, Chung-Ang University, Seoul 06974, Korea; MDPI , 2019 Dec; 8(12): 1620.
2. Prockop D.J., Kota D.J., Bazhanov N., Reger R.L. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J. Cell. Mol. Med. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x.
3. Stroncek D.F., Sabatino M., Ren J., England L., Kuznetsov S.A., Klein H.G., Robey P.G. Establishing a bone marrow stromal cell transplant program at the National Institutes of Health Clinical Center. Tissue Eng. Part B Rev. 2014;20:200–205. doi: 10.1089/ten.teb.2013.0529
4. Ramalho-Santos M., Yoon S., Matsuzaki Y., Mulligan R.C., Melton D.A. “Stemness”: Transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530.
5. Li L., Neaves W.B. Normal stem cells and cancer stem cells: The niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986.
6. Lin R.Z., Chang H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008;3:1172–1184. doi: 10.1002/biot.200700228.
