Cultivating spheroids from primary cancers and cancer stem cells
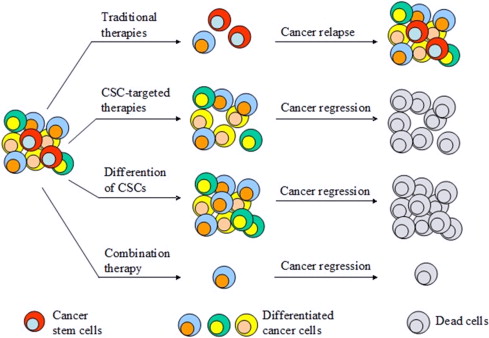
Cancer stem cells constitute a fraction of cancer cells, that are able to self renew, and differentiate to generate entire tumor structures. These cells were discovered in the 1990s, in hemopoietic cancers, and subsequently reported to be present in solid tumors as well as in gliomas, colon cancers and breast cancers (1-3). These discoveries propelled the advancement of culturing methods to produce solid tumors in vitro especially spheroid culturing techniques. It is of clinical significance to identify how to treat or eradicate cancer stem cells, as they play role in chemoresistance and cancer metastasis (4-5).
General protocol for tumor derived spheroids
There are varying sources of cells to produce tumor-derived spheroids. Most culturing techniques depend upon the innate ability of cancer stem cells to form in to spherical structures when in serum free media. The desired cancerous tissue is dissociated mechanically or via enzymatic reactions, then subsequently subjected to filtration or flow cytometry. The resulting single cells are suspended in a serum free media accompanied with growth factors (epidermal growth factors and fibroblast growth factors), and then cultivated on low attachment plates such as BIOFLOAT well plates, that are coated with a specialized polymeric coating that renders the surface cell repellant, thus facilitating cell-cell assembly (6).
Culturing parameters that can affect the quality of spheroids
Using these general protocols, researchers have been successful at isolating and propagating cancer cells with stem cell like properties, albeit the varying parameters calls for careful analysis of the results. For instance, cell density can affect stem cell like properties of cancer cells in that, higher cell density, can lead to cell aggregation and thus affect the clonal conditions necessary for cell growth. Numbers of passages also play a role as the initial cell number that is necessary for spheroid formation may also include transient amplifying cells. Spheroid size is also another factor that can affect cell growth and proliferation rate. As variations of these factors can determine the quality and translatability of the resulting spheroids, they must be carefully considered when comparing results from different spheroids of different cancer cells (6-7).
References
1. Lapidot T, Sirard C, Vormoor Jet al. A cell initiating human acute myeloidleukaemia after transplantation into SCID mice. Nature 1994; 367: 645–8.13
2. Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 2011; 17: 313–9.14
3. Singh SK, Clarke ID, Terasaki Met al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63: 5821–8.
4. Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug dis-covery. Nat Rev Drug Discov 2009; 8: 806–23.19
5. Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells–what challenges do they pose? Nat Rev Drug Discov 2014; 13: 497–512.
6. Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017 Mar; 108(3):283-289.
7. Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 2011;8:486–98
8. Han, Lu & Shi, Sanjun & Gong, Tao & Zhang, Zhi-Rong & Sun, Xun. (2013). Cancer stem cells: therapeutic implications and perspectives in cancer therapy. Acta Pharmaceutica Sinica B. 3. 65–75.
