Advancing Parkinson’s research with 3D cell cultures
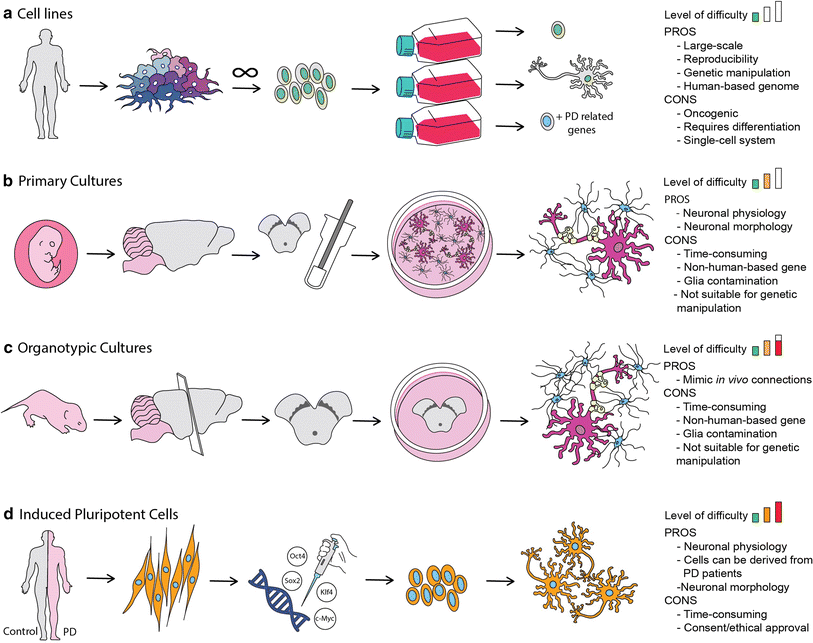
In the recent years most drugs designed to treat neurodegenerative disorders, have failed to produce desired effects at the clinical trial phase. This points towards a strong inability in conventional 2D culture assays, and animal models to replicate human pathophysiology, and thus yielding untranslatable data. In this regard, 3D cell cultures have enabled to bridge the gap, by providing platforms that are able to mimic cellular environments, neuronal and glial interactions and effects of blood flow in the brain and thereby, delivering predictive data that are useful for clinical trails (1).
Neurodegenerative diseases
Neurodegenerative diseases are chronic, progressive diseases characterized by the decline of brain function, and eventual loss of neuronal activity causing cognitive deficits and loss of motor function. Presently they have no cure, and has an adverse impact on for affected individuals and society, quality of life resulting in an ever-increasing burden on global healthcare systems (2).
Parkinson’s disease (PD)
Primarily identified as a movement disorder, parkinson’s is characterized by loss of deep grey matter, due to the loss of neurons in the substantia niagra, resulting in dopamine deficiency in the basal ganglia. It is known to affect approximately 1-2% persons over the age of 65 worldwide. The symptoms are bradykinesia, rigidity, resting tremors, and posture instability accompanied by non-motor symptoms such as sleep disturbances, constipation, dementia, and cognitive and olfactory deficits. Tissue pathology reveals, elevated levels of protein inclusions known as Lewy bodies or Lewy neurites within the neuronal cell body and processes respectively. Presently molecular etiology of PD remains unknown, and consequently therapeutic strategies are limited to managing the motor-related symptoms (3). Most drugs that performed well during pre clinical stages, were unsuccessful during the phase 2 and 3 clinical trials. This is mostly due to the lack of understanding of the molecular etiology of PD, caused by lack of representative models. Therefore experimental tools such as 3D cultures are in dire need to facilitate the progress in understanding PD, and discovery of effective therapeutic measures.
3D cell culture models for PD studies
The Human neuroblastoma cells (SH-SY5Y) are a popular cell line used for PD, as it is able produce doperminergic neurons typical of PD. 3D models of SH-SY5Y 3D models are developed by culturing these cells on a 3D matrix in DMEM media. Once differentiated the cells are exposed to recombinant α-synclein to develop tissue state typical of PD (4).
Lund human mesencephallic (LUHMES) is an immortalized cell line that is also used for PD research. These cells are able to recreate a physiologically relevant system with the added advantage of large scale cultures with good batch to batch consistency (5). The past decade, LUHMES cells were utilized as a 3D cell culture platform for neurotoxicity studies using the gyratory shaking technique, where cells are shaken in a continuous manner, facilitating the formation of spheroid aggregates, with astrocytes, neurons, oligodendrocytes and microglia. This system led to the formation of a phenotype that is similar to primary neurons, accompanied by natural extracellular matrix formation, and maturation of systems with myelination and synaptogenesis of astrocytes, neurons and oligodendrocytes (6). Other 3D models available include 3D human neuroectoderman spheres (hNES) that were used to develop a high throughput model using a microfluidic bioreactor, and human midbrain organoid models developed from iPSCs (7-8). With a wide range of different models, 3D cultures continue to be an essential tool for target validation, and bridging the gap between animal models and human, and ultimately in the development of personalized treatment options.
References
- Slanzi A, Iannoto G, Rossi B, Zenaro E and Constantin G (2020) In vitroModels of Neurodegenerative Diseases. Cell Dev. Biol. 8:328.
- Heemels, M.-T. (2016). Neurodegenerative diseases. Nature539:179. doi: 10.1038/539179a
- Poewe, W., Seppi, K., Tanner, C. M., Halliday, G. M., Brundin, P., Volkmann, J., et al. (2017). Parkinson disease. Rev. Dis. Primers3:17013. doi: 10.1038/nrdp.2017.13
- Xicoy, H., Wieringa, B., and Martens, G. J. (2017). The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Neurodegener.12:10.
- Harischandra, D. S., Rokad, D., Ghaisas, S., Verma, S., Robertson, A., Jin, H., et al. (2019). Enhanced differentiation of human dopaminergic neuronal cell model for preclinical translational research in Parkinson’s disease. Biophys. Acta Mol. Basis Dis.1866:165533.
- Smirnova, L., Harris, G., Delp, J., Valadares, M., Pamies, D., Hogberg, H. T., et al. (2016). A LUHMES 3D dopaminergic neuronal model for neurotoxicity testing allowing long-term exposure and cellular resilience analysis. Toxicol.90, 2725–2743.
- Kane, K. I. W., Moreno, E. L., Hachi, S., Walter, M., Jarazo, J., Oliveira, M. A. P., et al. (2019). Automated microfluidic cell culture of stem cell derived dopaminergic neurons. Rep.9:1796.
- Smits, L. M., Reinhardt, L., Reinhardt, P., Glatza, M., Monzel, A. S., Stanslowsky, N., et al. (2019). Modeling Parkinson’s disease in midbrain-like organoids. NPJ Parkinsons Dis.5:5.
- Lopes, Fernanda & Bristot, Ivi & Motta, Leonardo & Parsons, Richard & Klamt, Fábio. (2017). Mimicking Parkinson’s Disease in a Dish: Merits and Pitfalls of the Most Commonly used Dopaminergic In Vitro Models. NeuroMolecular Medicine. 19. 10.1007/s12017-017-8454-x.
