How Entering the Third Dimension of Cell Culture is Transforming Biological Research
Cell-based assays are one of the important pillars of the drug discovery process to provide a simple, fast, and cost-effective tool to avoid cost-intensive animal testing. To date, the traditional two-dimensional (2D) cell monolayers are the preferred method to culture cells due to their ease of utility and affordability (1).
Without any doubt, 2D cell culture is a valuable method for cell-based studies, but its limitations have been increasingly recognized. One of the major drawbacks of 2D cell culture is that it does not adequately reproduce the natural 3D environment of cells. As a result, 2D cell culture tests have misled nonpredictive data for in vivo responses in expensive phases of clinical research and drug discovery. Therefore, it is crucial to establish in vitro cell-based systems that can more realistically mimic in vivo cell behaviors and provide more reliable results for in vivo tests (2).
Advantages of 3D cell culture
Cellular processes and events in a 3D culture very closely mimic the physiological conditions and have distinct advantages over 2D culture conditions. While in traditional 2D cultures cells are grown as a monolayer on either glass or tissue culture surfaces, in 3D cell cultures cells grow as 3D aggregates/spheroids using either a scaffold/matrix or in a scaffold-free Manner, like in BIOFLOAT™ plates.
In contrast to 2D monolayer culture, when grown in 3D culture systems cells form aggregates, which more closely mimic the cell-cell and cell–ECM interactions, thereby replicating the natural environment found in vivo (3). Additionally, 3D spheroids consist of cells in various stages, usually, proliferating, quiescent, apoptotic, hypoxic, and necrotic cells (4,5), suggesting that the behavior of 3D-cultured cells reflects the in vivo cellular responses. The additional dimensionality of 3D cultures is the crucial feature that leads to the differences in cellular responses as it influences both the spatial organization of the cell surface receptors engaged in interactions with surrounding cells and provides physical constraints to cells. Both features affect the signal transduction from the outside to the inside of cells, and ultimately influence gene expression and cellular behavior.
Overview of 3D cell culture techniques
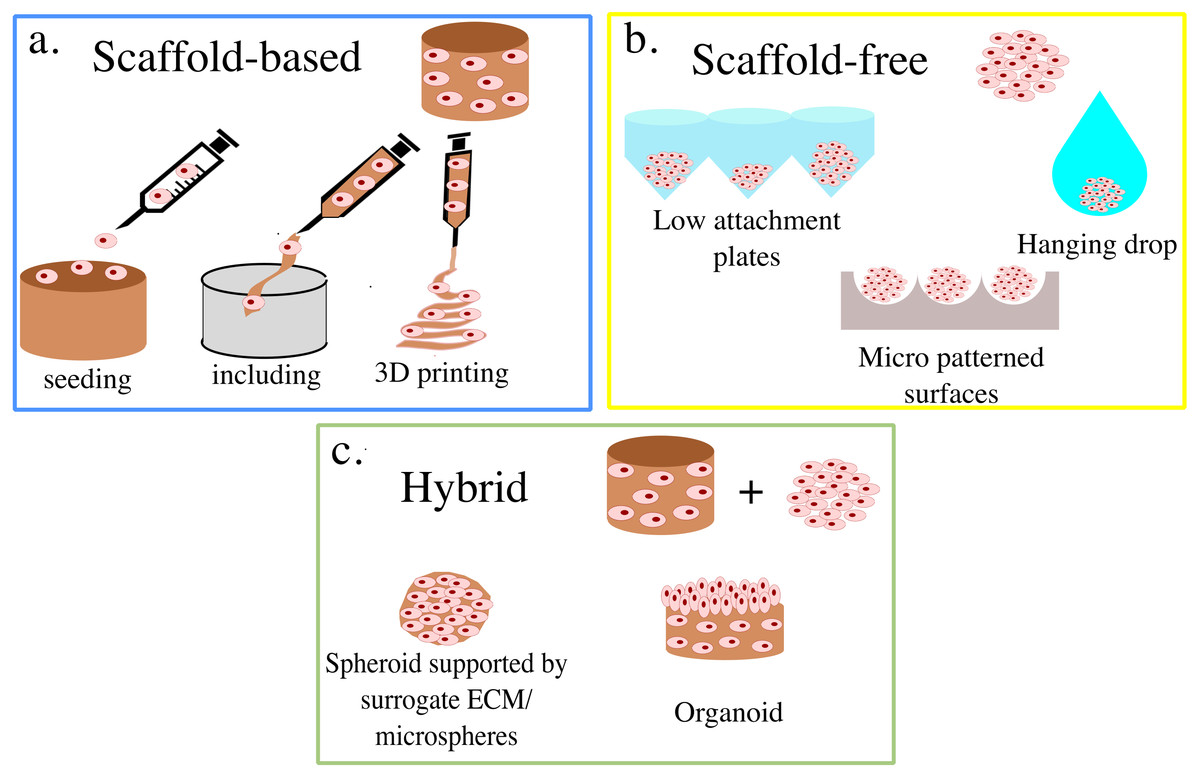
The choice of 3D cell culture technique depends on many parameters, including the nature of the cells and the final aim of the study. It’s crucial to evaluate the parameters before choosing the most relevant 3D cell culture technique. 3D cell culture techniques are broadly classified as Scaffold-based or non-scaffold-based techniques. In the scaffold-based technique, cells are grown in the presence of two major types of support scaffolds – hydrogel-based and polymeric-based.
Hydrogels are polymer networks extensively swollen with water and the cells can be embedded in these hydrogels or simply coated at the surface (2D cell culture). On the contrary, in polymeric-based scaffolds cells are cultivated in the presence of fibers or sponge-like structures made of polystyrene or polycaprolactone. Scaffold-free techniques allow the cells to self-assemble to form non-adherent cell aggregates called spheroids. The convenience of handling cells in vitro while gaining results that reflect in vivo conditions and avoiding ethical concerns about animal models make the 3D cell culture technique increasingly popular among researchers (6). The future indeed looks exciting with more complex and advanced technologies like 3D cell
bioprinting with wide applications in medicine like skin grafting having the potential to evolve as an indispensable tool for drug discovery and toxicity studies (7).
References
1. Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015 Mar 11;16(3):5517-27. doi: 10.3390/ijms16035517. PMID: 25768338; PMCID: PMC4394490.
2. Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro–a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005 Oct;15(5):405-12. doi: 10.1016/j.semcancer.2005.06.009. PMID: 16055341.
3. Shield K, Ackland ML, Ahmed N, Rice GE. Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecol Oncol. 2009 Apr;113(1):143-8. doi: 10.1016/j.ygyno.2008.11.032. Epub 2009 Jan 10. PMID: 19135710.
4. Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol. 2005 Oct;15(5):365-77. doi: 10.1016/j.semcancer.2005.05.002. PMID: 15975824.
5. Khaitan D, Chandna S, Arya MB, Dwarakanath BS. Establishment and characterization of multicellular spheroids from a human glioma cell line; Implications for tumor therapy. J Transl Med. 2006 Mar 2;4:12. doi: 10.1186/1479-5876-4-12. PMID: 16509995; PMCID: PMC1420330.
6. Maltman DJ, Przyborski SA. Developments in three-dimensional cell culture technology aimed at improving the accuracy of in vitro analyses. Biochem Soc Trans. 2010 Aug;38(4):1072-5. doi: 10.1042/BST0381072. PMID: 20659006.
7. Zhu W, Ma X, Gou M, Mei D, Zhang K, Chen S. 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol. 2016 Aug;40:103-112. doi: 10.1016/j.copbio.2016.03.014. Epub 2016 Apr 1. PMID: 27043763.