Three-dimensional Cell Culture: A Look into the Future
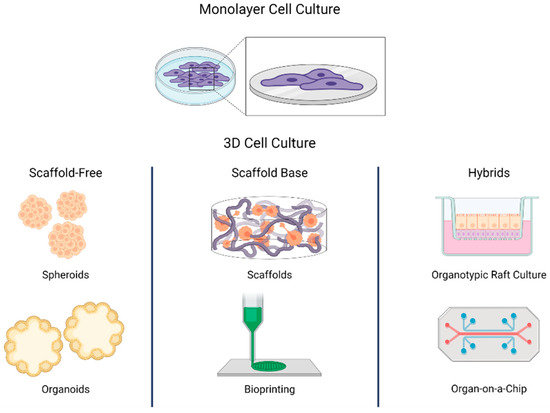
Two-dimensional (2D) cell cultures have been used by researchers since the early 1900s, but we are well aware of how growing cells on planar surfaces has some drawbacks. Cells grown in vitro in 2D space don’t behave like cells found in vivo and lack critical cell-cell and cell-matrix interactions which are fundamental for cell form, function, and response to external stimuli. This drawback results in unreliable data for translational research, which is why three-dimensional (3D) cell culture techniques have become more popular recently (1,2).
Current 3D techniques and models
Scaffolds are porous supports that regulate cell growth and enhance cell-cell signaling. 3D multi-layer cell assembly is successfully possible using scaffolds having a fundamental influence on cell differentiation and proliferation. As they grow within the scaffold, mature cells end up interacting with each other. They will eventually turn into structures that closely reproduce the tissues from which the cells initially originated. Scaffolds can be hydrogels, membranes, and 3D matrices, and materials such as metals, glasses, and ceramics can also constitute a scaffold.

However, scaffold-free cell culture techniques, which don’t rely on such solid supports can be also used to generate 3D cell culture models. These include forced-floating methods, hanging drop methods, and agitation-based methods. When referring to 3D cell culture systems, the terms spheroid and organoid have been commonly used. An example of a spheroid is constituted by the classical multicellular tumor spheroid model (MCTS) where the spherical geometry of the cell aggregate is characterized by an external proliferating region and an internal quiescent zone, which surrounds a necrotic core, thereby mimicking the cellular heterogeneity observed in solid tumors (3).
On the other hand, organoids are classified as tissue- or stem-cell-derived. Organoids representing different organ systems, such as the brain, liver, thymus, thyroid, lung, pancreas, intestines, and heart, have successfully been generated to study organ development, perform drug discovery/screening, model diseases, and perform toxicity testing (4).
What to expect in the future
Spheroid and organoid 3D cultures are advanced methods compared to monolayer cultures but they still fail to represent the complete architecture of in vivo tissues including the vasculature and interstitial fluid flow. The more advanced ‘organ-on-a-chip’ (OOC) technology may have the potential to address these limitations. The OOC technology is based on polymeric devices that represent the amalgamation of microfluidics and tissue engineering and are able to replicate in vivo vascularized tissues (5,6).
Along with OOC, 3D bioprinting is an additive manufacturing process to create a 3D structure through controlled and automated deposition of a biocompatible material or ‘bioink’. This technology is advanced enough to accurately construct complex tissue structures mimicking the actual in vivo architecture (7,8). The improvement in 3D culture technology encompasses more physiological and tissue-specific microenvironments overcoming the drawbacks observed in 2D cell culture models although there are still practical challenges in the mass adoption of 3D cell cultures because of high costs and elaborated procedures.
References
1. Lovitt CJ, Shelper TB, Avery VM. Advanced cell culture techniques for cancer drug discovery. Biology (Basel). 2014 May 30;3(2):345-67. doi: 10.3390/biology3020345. PMID: 24887773; PMCID: PMC4085612.
2. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012 Mar 20;21(3):309-22. doi: 10.1016/j.ccr.2012.02.022. PMID: 22439926.
3. Worthington P, Drake KM, Li Z, Napper AD, Pochan DJ, Langhans SA. Beta-Hairpin Hydrogels as Scaffolds for High-Throughput Drug Discovery in Three-Dimensional Cell Culture. Anal Biochem (2017) 535:25–34. doi: 10.1016/j.ab.2017.07.024
4. Karkampouna, Sofia & La Manna, Federico & Benjak, Andrej & Kiener, Mirjam & Menna, Marta & Zoni, Eugenio & Grosjean, Joël & Klima, Irena & Garofoli, Andrea & Bolis, Marco & Vallerga, Arianna & Theurillat, Jean-Philippe & de filippo, maria rosaria & Genitsch, Vera & Keller, David & Booij, Tijmen & Stirnimann, Christian & Eng, Kenneth & Sboner, Andrea & Kruithof-de Julio, Marianna. (2021). Patient-derived xenografts and organoids model therapy response in prostate cancer. Nature Communications. 12. 10.1038/s41467-021-21300-6.
5. Huang K, Boerhan R, Liu C, Jiang G. Nanoparticles Penetrate into the Multicellular Spheroid-on-Chip: Effect of Surface Charge, Protein Corona, and Exterior Flow. Mol Pharm. 2017 Dec 4;14(12):4618-4627. doi: 10.1021/acs.molpharmaceut.7b00726. Epub 2017 Nov 13. PMID: 29096441.
6. Shirure VS, Bi Y, Curtis MB, Lezia A, Goedegebuure MM, Goedegebuure SP, et al. Tumor-On-a-Chip Platform to Investigate Progression and Drug Sensitivity in Cell Lines and Patient-Derived Organoids. Lab Chip (2018) 18(23):3687–702. doi: 10.1039/C8LC00596F
7. Fong ELS, Toh TB, Yu H, Chow EK. 3D Culture as a Clinically Relevant Model for Personalized Medicine. SLAS Technol. 2017 Jun;22(3):245-253. doi: 10.1177/2472630317697251. Epub 2017 Mar 9. PMID: 28277923.
8. Deo KA, Singh KA, Peak CW, Alge DL, Gaharwar AK. Bioprinting 101: Design, Fabrication, and Evaluation of Cell-Laden 3D Bioprinted Scaffolds. Tissue Eng Part A. 2020 Mar;26(5-6):318-338. doi: 10.1089/ten.TEA.2019.0298. PMID: 32079490; PMCID: PMC7480731.