Generating large scale primary liver spheroids
Liver spheroids are successful as a physiologically relevant 3D cell culture model, with accurate representation of in-vivo like structure and function. In addition these 3D platforms are compatible with most high throughput, and high content screening systems, thus proving to be a vital tool in drug and toxicology screens for treatment of liver diseases. Spheroids are self-assembled aggregates, of uniform shape and structure. They can be rapidly generated, are relatively low tech, with long-term stability and viability of cultures, further highlighting their value as a reliable research platform.
Stirring bioreactors
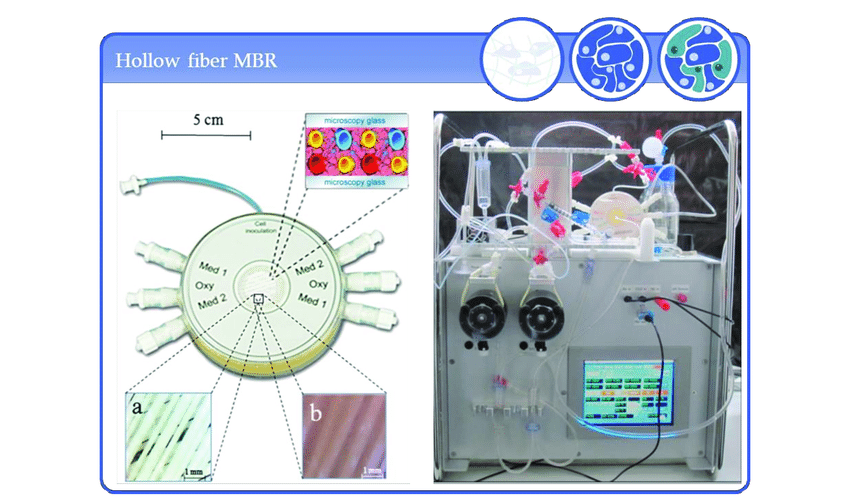
For large-scale applications primary human spheroids are generated in non-static, stirring bioreactors. Bioreactors are dynamic cell culture systems that can be monitored remotely; with a close regulation of cell culture parameters to generate the best microenvironment for liver cells (1). These culture parameters comprise of medium flow, temperature, pH, gas tension, glucose metabolism and lactate production. Hepatic functional viability of the culture is determined by urea and albumin production and ammonia detoxification. As the parameters can be regulated online, real time monitoring of cell viability is ensured (2).
PHH spheroids in bioreactors
Primary human hepatocytes (PHH) cultured in this manner were found to form functional bile canaliculi, with stable levels of albumin secretion and urea synthesis for 14 days, confirming the functional representation of the 3D culture as a reliable liver model. The cultivated spheroids were also found to consist of intact RNA expression of wide range of phase 1 and phase 2 enzymes. Furthermore, co-culturing PHH with mesenchymal cells from bone marrow further indicated increased cytochrome p450 levels further affirming the accuracy of the system as an efficient 3D culture platform. Regardless of the potential of these results, bioreactors do require large number of cells, and results in spheroids of different sizes, which can introduce complications in data analysis, and affect its high throughout compatibility. Therefore further progress is required to optimize bioreactors system, to produce homogenous uniform spheroids, thus making it more adaptable for high throughput screens of different conditions (3,4).
References
1. Farzaneh, Z., Abbasalizadeh, S., Asghari-Vostikolaee, M. H., Alikhani, M., Cabral, J. M. S., and Baharvand, H. (2020). Dissolved oxygen concentration regulates human hepatic organoid formation from pluripotent stem cells in a fully controlled bioreactor. Biotechnol. Bioeng. 117, 3739–3756.
2. Rowe, C., Gerrard, D. T., Jenkins, R., Berry, A., Durkin, K., Sundstrom, L., et al. (2013). Proteome-wide analyses of human hepatocytes during differentiation and dedifferentiation. Hepatology 58, 799–809.
3. Tostoes, R. M., Leite, S. B., Serra, M., Jensen, J., Bjorquist, P., Carrondo, M. J., et al. (2012). Human liver cell spheroids in extended perfusion bioreactor culture for repeated-dose drug testing. Hepatology 55, 1227–1236.
4. Rebelo SP, Costa R, Silva MM, Marcelino P, Brito C, Alves PM. Three-dimensional co-culture of human hepatocytes and mesenchymal stem cells: improved functionality in long-term bioreactor cultures. J Tissue Eng Regen Med. 2017 Jul;11(7):2034-2045.
5. Altmann, Brigitte & Grün, Christoph & Nies, Cordula & Gottwald, Eric. (2020). Advanced 3D Cell Culture Techniques in Micro-Bioreactors, Part II: Systems and Applications. Processes. 9. 21. 10.3390/pr9010021.
