ALZHEIMER’S BREAKTHROUGHS WITH 3D CELL CULTURES
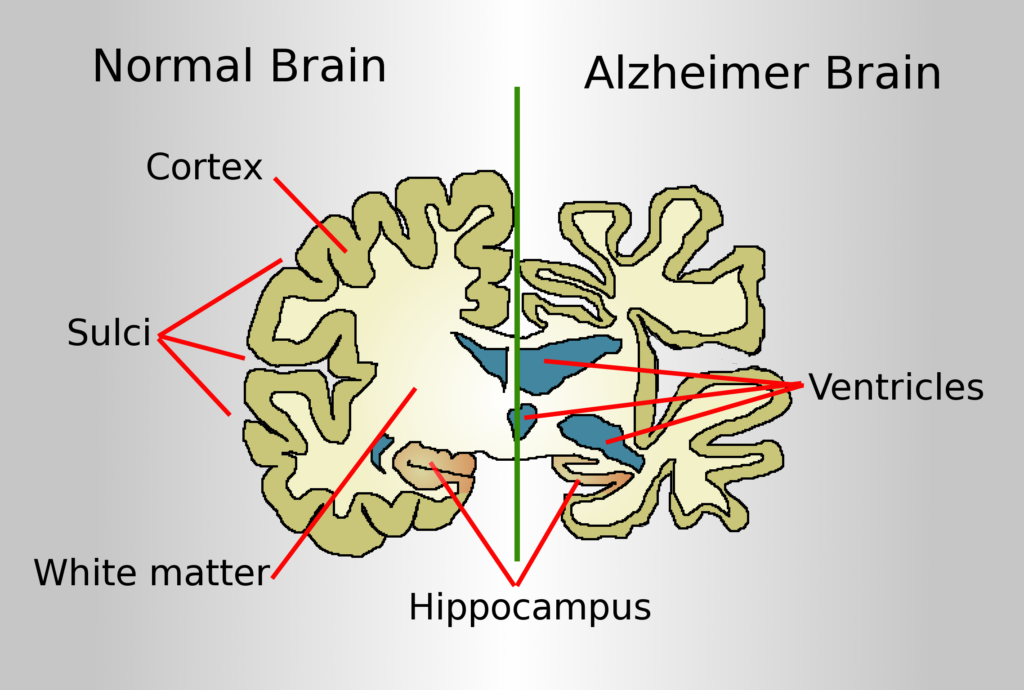
The limitations of 2D cultures have paved the way for the development of 3D cell culture models of Alzheimer’s disease (AD). AD is a neurodegenerative disorder comprised of progressive decline of cognitive abilities and behaviour leading to a poor quality of life. The etiology and pathogenesis of AD is partially explained by the amyloid cascade hypothesis, in which accumulation of amyloid β (Aβ) protein causes a detrimental turn of events leading to the accumulation of neurofibfillary tangles (NFTs), neuronal cell death and dementia. However, much of pathogenesis of AD still remains unknown due to the lack of physiologically relevant in-vivo and in-vitro models. The advances made in 3D cultures however, provides a more comprehensive system to investigate the multifactorial etiology of AD, while overcoming the inadequacies found in traditional monolayer cultures and animal models (1-3).
Development of 3D culture models for AD
The first 3D culture model was developed from immortalized human neural stem cells (ReN) containing genetic mutations typically associated with AD. The cultures exhibited senile plaques along with NFTs, previously not recapitulated in 2D cell cultures and animal models. The differentiated ReN cells when cultivated in a scaffold based 3D system, prevented the diffusion of Aβ, thus encouraging the aggregation and formation of Aβ plaques (4).
Neurospheroids and Cerebral organoids
Cerebral organoids are the latest step up towards developing an accurate in-vitro representation of AD. Human ReN cells were once again utilized to produce neurospheroids within low attachment plates. Following 8 weeks of cultures these spheroids exhibited Aβ plaques together with hyperphosphorylated tau proteins, both being consistent with the pathogenesis of AD (5).
Mimicking the blood brain barrier deficits in AD

One of the limitations of conventional organoids is the occurrence of necrotic regions, caused by limited oxygen supply and nutrient diffusion. Vascularized human cortical organoids (vhCOs) had the advantage of overcoming these limitations by linking a perfusion system consistent with cortical vasculature, to the cerebral organoids, to allow for proper oxygen and nutrient supply (6). Although this models lacks blood cells, the use of drugs that regulates the permeability of the blood brain barrier (BBB) could yield a realistic AD model to investigate the trafficking of Aβ as well as other molecules. Additionally other microfluidics 3D cultures systems has aided in creating a representative platform of blood flow typically seen in AD (7,8). Such models are promising avenues for identifying patho-physiological mechanisms of BBB dysfunction that is typical of AD, as well as being a standardized platform for drug screens. Furthermore 3D neurospheroids developed from iPSCs, has helped to understand individual genetic variations of AD thus providing a promising platform to perform more accurate drug screens, for personalized therapy (9).
References
1. Scheltens, P., Blennow, K., Breteler, M. M. B., De Strooper, B., Frisoni, G. B., Salloway, S., et al. (2016). Alzheimer’s disease. Lancet 388, 505–517.
2. Reitz, C. (2012). Alzheimer’s disease and the amyloid cascade hypothesis: a critical review. Int. J. Alzheimers Dis. 2012:369808.
3. Iqbal, K., and Grundke-Iqbal, I. (2010). Alzheimer’s disease, a multifactorial disorder seeking multitherapies. Alzheimers Dement. 6, 420–424.
4. Choi, S. H., Kim, Y. H., Hebisch, M., Sliwinski, C., Lee, S., D’avanzo, C., et al. (2014). A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 515, 274–278.
5. Jorfi, M., D’avanzo, C., Tanzi, R. E., Kim, D. Y., and Irimia, D. (2018). Human neurospheroid arrays for in vitro studies of Alzheimer’s Disease. Sci. Rep. 8:2450.
6. Kelava, I., and Lancaster, M. A. (2016). Stem cell models of human brain development. Cell Stem Cell 18, 736–748.
7. Shin, Y., Choi, S. H., Kim, E., Bylykbashi, E., Kim, J. A., Chung, S., et al. (2019). Blood-brain barrier dysfunction in a 3D in vitro model of Alzheimer’s disease. Adv. Sci. 6:1900962.
8. Park, J., Wetzel, I., Marriott, I., Dreau, D., D’avanzo, C., Kim, D. Y., et al. (2018). A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 21, 941–951.
9. Lee, H. K., Velazquez Sanchez, C., Chen, M., Morin, P. J., Wells, J. M., Hanlon, E. B., et al. (2016). Three dimensional human neuro-spheroid model of Alzheimer’s disease based on differentiated induced pluripotent stem cells. PLoS One 11:e0163072.
10. Laredo, Fabio & Plebanski, Julia & Tedeschi, Andrea. (2019). Pericytes: Problems and Promises for CNS Repair. Frontiers in Cellular Neuroscience. 13.
