3D cell culture as a suitable model for respiratory syncytial virus
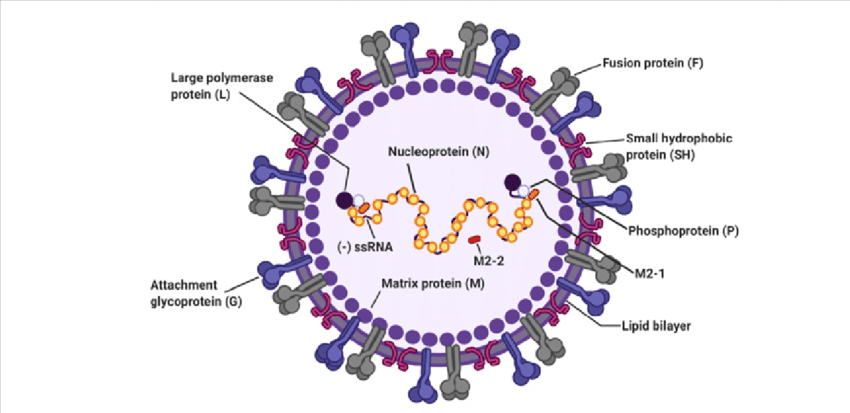
Respiratory Syncytial Virus (RSV) is one of the most common respiratory tract viruses with high mortality rate among infants below the age of 5. Within the adult population it can cause chronic disease and weaker immunity. RSV is mainly found within alveolar pneumocytes and bronchial and bronchiolar cells of the lower respiratory tract. It can cause aggravated mucus production and airway obstruction. Traditional 2D monolayer cultures have so far been unsuccessful at recapitulating RSV pathogenesis in-vivo, causing researchers to move towards developing 3D cell culture models that are suitable for RSV studies (1).
3D models developed for RSV
3D aggregates and airway organoids are primarily used as the main 3D models for studies investigating RSV pathogenesis, potential therapeutics and host-immune response. To this end, lung bud organoids grown from human pluripotent stem cells (HPSc) were developed to examine its ability to recapitulate human lung infection caused by RSV. The organoids indicated disease characteristics similar to that observed in response to viral infection in vivo (2).
Furthermore, 3D spheroids of alveolar epithelial type II cells (A549) were also found effective at mimicking the disease features of RSV. The aggregates allowed viral entry and propagation, and exhibited clinical features of the disease such as excessive mucin production and large syncytia formation typical of host cell response upon infection. A549 spheroid model thus serves as a prime example of the relevance of 3D spheroids as a platform for RSV related studies (3).
Much like in parainfluenza viruses, little progress has been made in vaccine development for RSV. 3D aggregates and organoids were utilised here again, in an attempt to identify potential therapeutics and vaccines. The EpiAirway 3D culture model infected with strains of RSV showed some success, in responding to the anti-viral compounds, which was confirmed to be the most effective treatment option in reducing the viral load (4). Similar studies performed with 3D cultures of human airway epithelium cells (HuAECs) also supported the relevancy of 3D cultures as a effective model for investigating RSV therapeutics (5).These studies along with many others continue to confirm 3D aggregates and organoid models as the most effective platform to recapitulate viral pathogenesis, clinical features of RSV and provide a suitable platform to test the efficacy of possible anti-viral therapeutics and vaccines (6).
References
1. Gu, J.; Korteweg, C. Pathology and Pathogenesis of Severe Acute Respiratory Syndrome. Am. J. Pathol. 2007, 170, 1136–1147
2. Chen, Y.-W.; Huang, S.X.; De Carvalho, A.L.R.T.; Ho, S.-H.; Islam, M.N.; Volpi, S.; Notarangelo, L.D.; Ciancanelli, M.; Casanova, J.-L.; Bhattacharya, J.; et al. A three-dimensional model of human lung development and disease from pluripo- tent stem cells. Nat. Cell Biol. 2017, 19, 542–549.
3. Saleh, F.; Harb, A.; Soudani, N.; Zaraket, H. A three-dimensional A549 cell culture model to study respiratory syncytial virus infections. J. Infect. Public Health 2020, 13, 1142–1147.
4. Mcallister,N.V.Investigationof3DPrimaryHumanAirwayCellCultureasaViableandSuccessfulModelforStudyofRespiratory Syncytial Virus Infection and Antiviral Drug Treatment. Master’s Thesis, Harvard Extension School, Cambridge, MA, USA, 2020.
5. Mirabelli, C.; Jaspers, M.; Boon, M.; Jorissen, M.; Koukni, M.; Bardiot, D.; Chaltin, P.; Marchand, A.; Neyts, J.; Jochmans, D. Differential antiviral activities of respiratory syncytial virus (RSV) inhibitors in human airway epithelium. J. Antimicrob. Chemother. 2018, 73, 1823–1829.
6. Deatly, A.M.; Lin, Y.-H.; McCarthy, M.; Chen, W.; Miller, L.Z.; Quiroz, J. Paramyxovirus Infection Mimics In Vivo Cellular Dynamics in Three-Demensional Human Bronchio-Epithelial Tissue-Like Assemblies; NASA Johnson Space Center: Houston, TX, USA, 2012.
7. Ibekwe, Titus & Kwaghe, Vivian & Habib, Zaiyad & Ibekwe, Perpetua. (2021). The Role of Antiviral Drugs in the Case-Management of Respiratory Syncytial Virus, Influenza and COVID-19.