What are the most commonly used cryoprotectants?
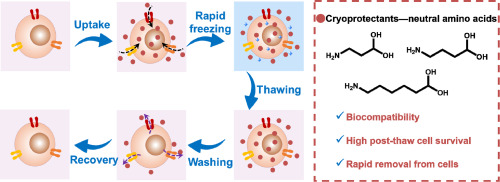
Cryoprotectants are crucial in storing biological samples at a deep cryogenic temperature. Cryoprotecting agents lower the melting point of water when dissolved in it and prevent ice formation by increasing the total concentration of all the solutes present in the sample and hence protect the cells. Since cryopreserved samples need to have the same level of viability upon recovery, cryoprotectants must penetrate into the cells and should have lower toxicity. This article discusses the most commonly used cryoprotectants used in life science research (1,2).
1. Dimethyl sulfoxide (DMSO)
The Russian scientist Alexander Zaytsevun synthesized DMSO in 1866. DMSO is the most commonly used cryoprotectant to protect cells. It is a cell-penetrating agent and protects biological samples from intracellular ice formation. It represents a cost-effective option with minor toxicity. However, DMSO can cause DNA methylation which in turn altered cells and histones thereby becoming DMSO’s major drawback (3).
2. Ethylene glycol
60% ethylene glycol is incapable of forming crystals when mixed with 40% water which makes it one of the ideal cryoprotectants. Ethylene glycol interferes with the hydrogen bonds in water and makes it difficult for the water molecules to bind. This prevents the formation of ice crystals and lowers the water’s freezing point. However, it is also toxic to some level causing gastrointestinal irritation, lung inflammation, and edema.
3. Glycerol
Glycerol is a colorless and odorless viscous liquid. It is a good kosmotropic agent and forms stable hydrogen bonds with water molecules. It contributes to the stability and structure of water-water interactions. This characteristic makes it difficult to form ice crystals (4).
4. Propylene Glycol
Propane-1, 2-diol is a non-irritating, colorless, odorless, synthetic organic compound. It is miscible with water, chloroform, and acetone. It has the property of an automotive anti-freeze.
5. Polymers
Non-diffusible synthetic polymers like polyvinyl alcohol, polyethylene glycol (PEG), and Hydroxyethyl starch are some selective cryoprotective agents used for biological samples. They have a good potential to decrease the size of ice crystals.
References
1. Wohnhaas, C.T., Leparc, G.G., Fernandez-Albert, F. et al. DMSO cryopreservation is the method of choice to preserve cells for droplet-based single-cell RNA sequencing. Sci Rep 9, 10699 (2019). https://doi.org/10.1038/s41598-019-46932-z
2. Meryman HT. Cryoprotective agents. Cryobiology. 1971 Apr;8(2):173-83. doi: 10.1016/0011-2240(71)90024-1. PMID: 5578883.
3. Verheijen M, Lienhard M, Schrooders Y, Clayton O, Nudischer R, Boerno S, Timmermann B, Selevsek N, Schlapbach R, Gmuender H, Gotta S, Geraedts J, Herwig R, Kleinjans J, Caiment F. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci Rep. 2019 Mar 15;9(1):4641. doi: 10.1038/s41598-019-40660-0. PMID: 30874586; PMCID: PMC6420634.
4. Zhang PQ, Tan PC, Gao YM, Zhang XJ, Xie Y, Zheng DN, Zhou SB, Li QF. The effect of glycerol as a cryoprotective agent in the cryopreservation of adipose tissue. Stem Cell Res Ther. 2022 Apr 8;13(1):152. doi: 10.1186/s13287-022-02817-z. PMID: 35395949; PMCID: PMC8994386.
