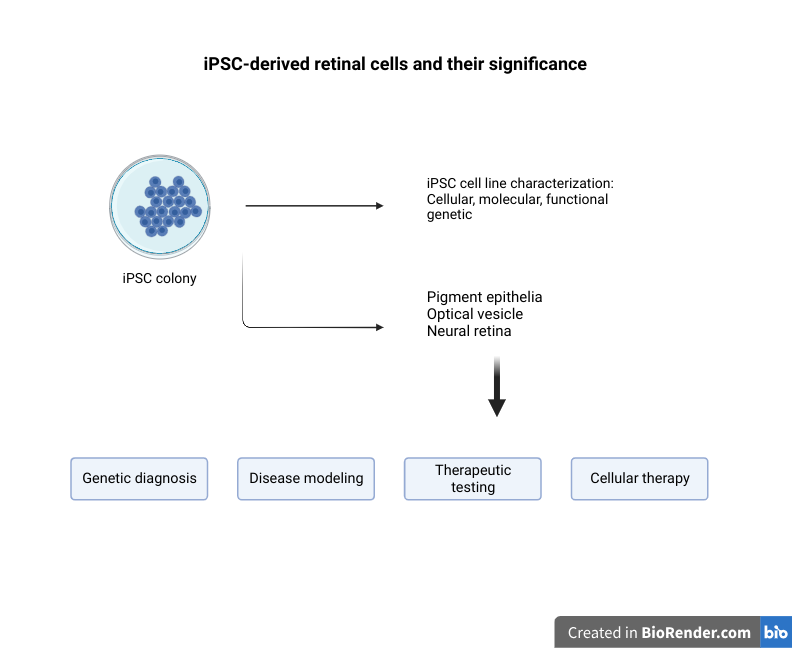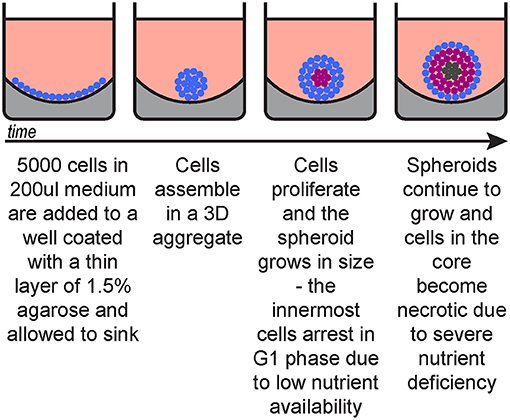
Three-dimensional skin spheroid cultures
Three-dimensional culture system provides unique platforms to study complex biological processes in vitro, particularly cell proliferation, differentiation, motility, and stress responses. While 3D spheroid cultivation methods are well established in certain tissue types such as mammary and colorectal epithelial cells, efficient 3D epithelial spheroid system for primary human cells are yet under developing phase, the challenge lies in keratinocytes sensitivity to surface detachment, as basal keratinocyte renewal is tightly regulated by cell attachment to the basement membrane (1,2)
Relative to two dimensional cultures, three-dimensional suspension cultures of epithelial cells more closely mimic the in vivo microenvironment regarding cell architecture, cell to matrix interaction, and osmosis exchange (3,4).
3D Human Neonatal keratinocytes
Primary normal human keratinocytes (NHKc) cells are isolated from the skin of a normal male. They are primary cells that provide a complete solution to propagate keratinocytes isolated from human skin. They rapidly undergo terminal differentiation and detachment induced cell death upon disconnection from the base membrane, thus constraining their use of 3D suspension culture models (4,5).
These cells also display prolonged keratinocyte renewal and a gene expression profile corresponding to cellular heterogeneity, quiescence and de differentiation. Spheroid derived NHKc enriched for P63 and K14 formed colonies and reassembled into multicellular spheroids during 3D suspension cultures (6).
REFERENCES
1. H. Green Terminal differentiation of cultured human epidermal cells Cell, 11 (1977), pp. 405-416
2. H. Wakita, M. Takigawa Activation of epidermal growth factor receptor promotes late terminal differentiation of cell-matrix interaction-disrupted keratinocytes J. Biol. Chem., 274 (1999), pp. 37285-37291
3. Guo, C.A. Jahoda An improved method of human keratinocyte culture from skin explants: cell expansion is linked to markers of activated progenitor cells Exp. Dermatol., 18 (2009), pp. 720-726
4. B.M. Kang, M.H. Kwack, M.K. Kim, J.C. Kim, Y.K. Sung Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay J, Invest. Dermatol., 132 (2012), pp. 237-239
5. G.S. Akerman, W.H. Tolleson, K.L. Brown, L.L. Zyzak, E. Mourateva, T.S. Engin, A. Basaraba, A.L. Coker, K.E. Creek, L. Pirisi Human papillomavirus type 16 E6 and E7 cooperate to increase epidermal growth factor receptor (EGFR) mRNA levels, overcoming mechanisms by which excessive EGFR signaling shortens the life span of normal human keratinocytes Cancer Res., 61 (2001), pp. 3837- 3843
6. B.M. Borena, E. Meyer, K. Chiers, A. Martens, K. Demeyere, S.Y. Broeckx, L. Duchateau, J.H. Spaas Sphere-forming capacity as an enrichment strategy for epithelial-like stem cells from equine skin Cell. Physiol. Biochem., 34 (2014), pp. 1291-1303