Organoids In Personalized Medicine
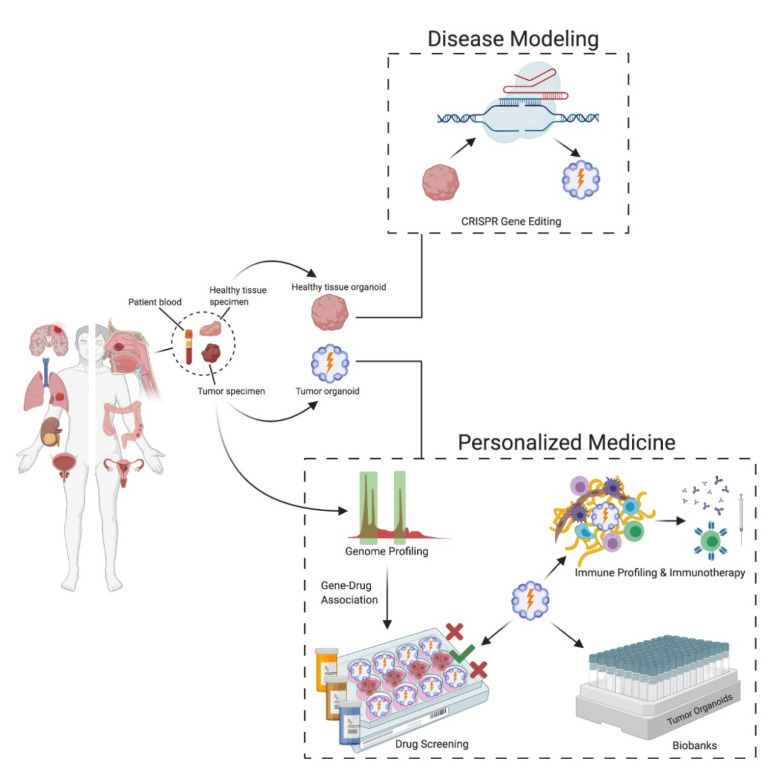
Organoids are self-organized 3D structures derived from adult or embryonic stem cells that are able to represent the structure and function of the derived tissue. They are able to accurately mimic tissue specific structure and function consistent with in-vivo tissue physiology (1).
Among the major advantages of organoids is its potential in personalized healthcare. Healthy and cancer tissues can be obtained from the same patient, creating a useful tool to predict drug response behaviour in a patient specific manner. This enables to identify drugs that specifically target cancer cells, thus encouraging the choice of less toxic compounds and decreasing the overall negative side effects commonly associated with cancer treatment (2).
To this end, liver organoid cultures from induced pluripotent stem cells (iPSCs) or healthy tissues are used to investigate hepatotoxicity of new experimental medicine (3,4). Furthermore, iPSC-derived kidney or cardiac organoids proved successful in toxicological studies. Additionally, patient derived tumoroids are also widely used to study genetic alterations in drug resistance mechanisms (5,6). Side by side, tumoroid and healthy organoid models, aids in identifying different protein expression patterns and mutations to fine-tune patient specific treatment (7).
Hurdles in using tumoroid models
Among the common drawbacks of the tumoroid models is the potential overgrowth of non-tumor components, which can interfere with culturing a homogenous model specific for particular genetic or phenotypic feature. For instance, in colorectal cancers, mutations in WNT signaling activation are a common occurrence (8). Thus to obtain WNT-mutated tumoroids it is important to use media without WNT and R-spondins (2). A balance must be struck therefore, between tumor and non-tumor cells to obtain a physiologically relevant tumoroid model. However, regardless of the meticulous culture conditions of organoid models, its advantages outweigh the drawbacks, and therefore will continue to be a reliable and reproducible research platform for both pathophysiological studies of various diseases, and propel the advancement of patient-specific treatments.
References
1. Zanoni, M., Cortesi, M., Zamagni, A. et al. Modeling neoplastic disease with spheroids and organoids. J Hematol Oncol 13, 97 (2020).
2. Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18: 407–18.
3. Meng Q. Three-dimensional culture of hepatocytes for prediction of druginduced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2010;6:733–46.
4. Katsuda T, Kawamata M, Hagiwara K, Takahashi R-U, Yamamoto Y, Camargo FD, et al. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell. 2017;20:41–55
5. Eder A, Vollert I, Hansen A, Eschenhagen T. Human engine human engineered heart tissue as a model system for drug testing. Adv Drug Deliv Rev. 2015;96:214–24.
6. Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–8.
7. Cristobal A, van den Toorn HWP, van de Wetering M, Clevers H, Heck AJR, Mohammed S. Personalized proteome profiles of healthy and tumor human colon organoids reveal both individual diversity and basic features of colorectal cancer. Cell Rep. 2017;18:263–74.
8. Sato T, Stange DE, Ferrante M, Vries RGJ, Van Es JH, Van Den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–72.
