Multicellular Tumor spheroids (MCT)
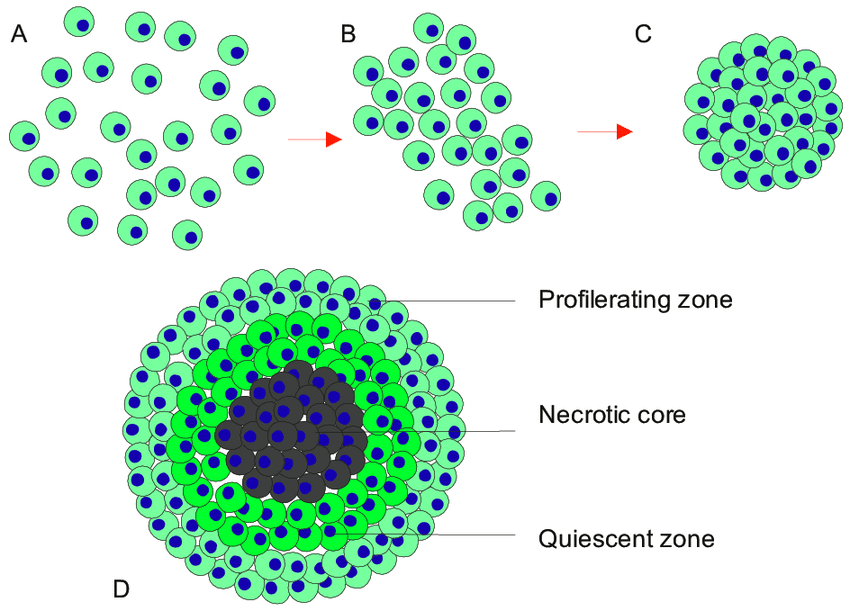
Multicellular tumor spheroids are fast becoming an essential tool in tumor related research, and drug screens. MCT can be defined as clusters of heterogeneous cells, formed by self-aggregation, closely packed with highly dense spheroids. These cells exhibit strong cell-cell and cell-extracellular matrix (ECM) communications representative of in vivo tumor physiology (1-2).
Methods for forming multicellular spheroids (MCT)
MCTs can be generated through scaffold free or scaffold incorporated culture methods. Scaffold free methods such as liquid overlay method, and hanging drop method are commonly utilized to generate MCTs. Liquid overlay method rely on the natural ability of cells to self-aggregate in the presence of a low adhesive, cell repellant surfaces. Hanging drop methods leans heavily upon surface tension and gravity to force the cells the self-aggregate. Additionally 3D bio printing, and microfluidic chambers are also employed as novel methods to generate MCTs (5-6).
Organoids are the main go to model in scaffold-based approaches to generate multicellular 3D structures. Cells are seeded on to artificial 3D scaffolds that resemble ECM structure (4). Unlike MCTs, organoids consist of organ specific cells cultivated from primary tissue or stem cells and are able to self renew, self organize and exhibit organ specific function. The scaffolding materials commonly employed includes Matrigel, or collagen, which is, used as an anchoring structure to support the growing organoids. The organoids have become vital in pathophysiological research of tumor growth and development (7,8).
Validity of MCTs
MCTs have proved to closely replicate in vivo solid tumor physiology with regards to replicating the main key features of a solid tumor. This includes
1) Structural organization
2) Oxygen, nutrient, and pH gradients
3) External proliferating zones (MCTs > 500μm in size)
4) Internal quiescent zone (MCTs > 500μm in size)
5) Necrotic core (MCTs > 500μm in size)
Aside from replicating key main features of solid tumors, MCTs also show in vivo like growth kinetics, metabolic rates and resistance to chemotherapy and radiation. MCTs have thus aided in our understanding of tumor biology and advanced the development of novel therapeutic options (9).
Limitations of MCTs
Despite its effectiveness there are a few concerns that can determine the reliability and reproducibility of a MCT model especially with regards to its use during the preclinical phase of drug screening. These concerns include 1) reproducibility in generating spheroids of uniform size and shape, 2) standardized validity testing that are available to assess the efficacy of MCTs growth and drug response, and 3) developing high throughput MCT cultures and reliable drug screening methods. All three concerns can determine MCTs’ reproducibility and effectiveness in commercial applications (9).
References
1. Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–45. 2.
2. Maddaly R, Paramesh V, Kaviya SR, Anuradha E, Paul Solomon FD. 3D cell culture systems: advantages and applications. J Cell Physiol. 2015;230:16–26.
3. Froehlich K, Haeger JD, Heger J, Pastuschek J, Photini SM, Yan Y, et al. Generation of multicellular breast cancer tumor spheroids: Comparison of different protocols. J Mammary Gland Biol Neoplasia. 2016;21(3–4):89–98.
4. Ricci C, Moroni L, Danti S. Cancer tissue engineering: new perspectives in understanding the biology of solid tumors: a critical review. Tissue Eng. 2013;1:4.
5. Tung YC, Hsiao AY, Allen SG, Torisawa YS, Mitchell HM, Takayama S. Highthroughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136(3):473–8.
6. Wu LY, Carlo DD, Lee LP. Microfuidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed Microdevices. 2008;10:197–202.
7. Seungil K, Sarah C, Ren XS, Nolan U, Natasha H, Emma JF, et al. Comparison of cell and organoid-level analysis of patient-derived 3D organoids to evaluate tumor cell growth dynamics and drug response. SLAS Discov. 2020;25(7):744–54.
8. Zarema G, Aleksei P, Catrin R, Albert R, Valeriya S. Promising applications of tumor spheroids and organoids for personalized medicine. Cancers. 2020;12:2727.
9. Han, S.J., Kwon, S. & Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int 21, 152 (2021).
10. Vadivelu, Raja & Kamble, Harshad & Shiddiky, Muhammad & Nguyen, Nam-Trung. Microfluidic Technology for the Generation of Cell Spheroids and Their Applications. Micromachines. 8. 94 (2017).
