Factors Affecting Reproducibility of Uniform Spheroids in 384 well Plates
In the recent years 3D cultures of tumour cell lines have become an essential tool in cancer research and drug discovery processes. 3D cultures when compared with traditional 2D monolayer cultures, are able to produce cell cultures bearing close resemblance to native physiological conditions (1). Tumour cell spheroids in particular, are able to replicate complex solid tumours with intact cell-cell interactions, proliferating zones, quiescent cells, diffusion gradients, multicellular resistance, and hypoxic regions, much like that observed in physiological conditions. Due to its ability to effectively recreate tumour like properties, spheroids are now being considered as the pathophysiologically and biochemically relevant model for drug screens and toxicity assays (2,3).
Adaptability in High Throughput assays
There are a wide range of standardised protocols developed to enable these tumour spheroids to be compatible with high throughout (HT)/high content screening (HTS). Among the common hindrances encountered in using spheroids in HTS, includes variability in spheroid size, shape and growth (1,3).
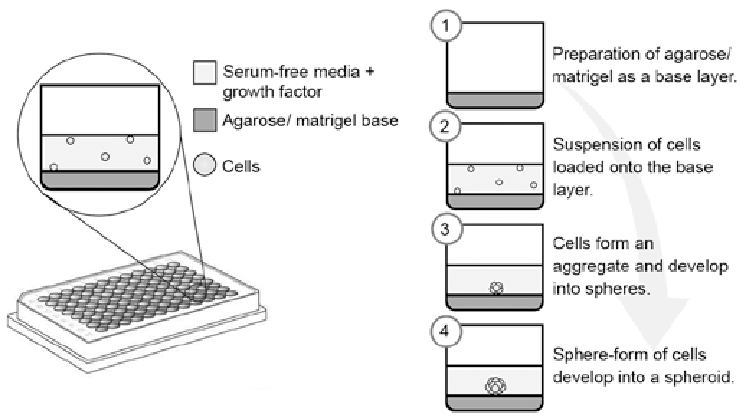
Liquid overlay method on agarose coated plates is a common technique employed for spheroid formation (3). Agarose based methods are inexpensive, low tech, and compatible with standard multi well plates as well as microtitre plates. Use of agarose coating on 96 and 384 well plates were successful at producing spheroids that can be used in HTS assays (2).
Hurdles in miniaturising assays for HTS
To reduce variability in spheroid size and shape, an additional step of coating cell aggregates with growth factor-reduced Matrigel was incorporated to much success (4). Another hurdle encountered when miniaturising assays for HTS, is edge effect in microtitre plates (5-6). Prolonged incubation periods results in accelerated evaporations of medium from the peripheral walls of the plates leading to inconsistent readouts (7). Additionally evaporation of medium also results in osmolality shifts in the medium that affects growth and metabolism of cells, and in turn affect the overall uniformity of the spheroids (6,7). Changes in spheroid shape and size can in turn cause significant alterations in drug response across assay plates.
To reduce medium evaporation, significant improvements in culture conditions are made that includes using embryo grade mineral oil as an alternative low cost approach. Other modifications include minor technical improvements such as the use of 0.75% filtered agarose solutions to increase the scalability of agarose overlay culturing method. These modifications offer the added benefit of requiring no additional equipment or consumables, and easy of use in already existing lab conditions in HTS laboratories. With the aid of on going technical advances in culture conditions, multi well spheroid cultures is sure to further improve and expand its use in HTS drug discovery (7).
References
1. Das, V.; Bruzzese, F.; Konečný, P.; et al. Pathophysiologically Relevant In Vitro Tumor Models for Drug Screening. Drug Discov. Today 2015, 20, 848–855.
2. Wenzel, C.; Riefke, B.; Gründemann, S.; et al. 3D High- Content Screening for the Identification of Compounds That Target Cells in Dormant Tumor Spheroid Regions. Exp. Cell Res. 2014, 323, 131–143.
3. LaBarbera, D. V.; Reid, B. G.; Yoo, B. H. The Multicellular Tumor Spheroid Model for High-Throughput Cancer Drug Discovery. Expert Opin. Drug Discov. 2012, 7, 819–830.
4. Li, Q.; Chen, C.; Kapadia, A.; et al. 3D Models of Epithelial- Mesenchymal Transition in Breast Cancer Metastasis: High- Throughput Screening Assay Development, Validation, and Pilot Screen. J. Biomol. Screen. 2011, 16, 141–154.
5. Berg, M.; Undisz, K.; Thiericke, R.; et al. Evaluation of Liquid Handling Conditions in Microplates. J. Biomol. Screen. 2001, 6, 47–56.
6. Berthier, E.; Warrick, J.; Yu, H.; et al. Managing Evaporation for More Robust Microscale Assays Part 1. Volume Loss in High Throughput Assays. Lab. Chip 2008, 8, 852–859.
7. Das V, Fürst T, Gurská S, Džubák P, Hajdúch M. Reproducibility of Uniform Spheroid Formation in 384-Well Plates: The Effect of Medium Evaporation. Journal of Biomolecular Screening. 2016;21(9):923-930.
8. Sankar, Prabu & Firdaus, Mohd & Muniandy, Kalaivani & Lian, Benedict & Ling, Phang & Hoe, Susan & Khoo, Alan & Mohana-Kumaran, Nethia. (2017). Modeling nasopharyngeal carcinoma in three dimensions (Review). Oncology Letters. 13.
9. Kamatar A, Gunay G, Acar H. Natural and Synthetic Biomaterials for Engineering Multicellular Tumor Spheroids. Polymers. 2020; 12(11):2506.