Effect Of Culture Methods On Multicellular Tumor (MCT) Spheroid Formation
MCTs can be cultivated in scaffold free or scaffold based 3D cultures. Scaffold based 3D cultures includes the use of a 3D artificial matrix or hydrogels as a anchorage for the cells, as well as facilitating the formation of cell-extracellular matrix (ECM) interactions. Both natural (gelatin, alginate, collagen and Matrigel) and synthetic compounds (PLGA, PCL, and PEG) are used to produce the scaffold. Natural polymers are preferred over synthetic compounds due to their biocompatibility. Synthetic compounds on the other hand offer better availability, and production in large quantities and can be customized for various applications (1-3).
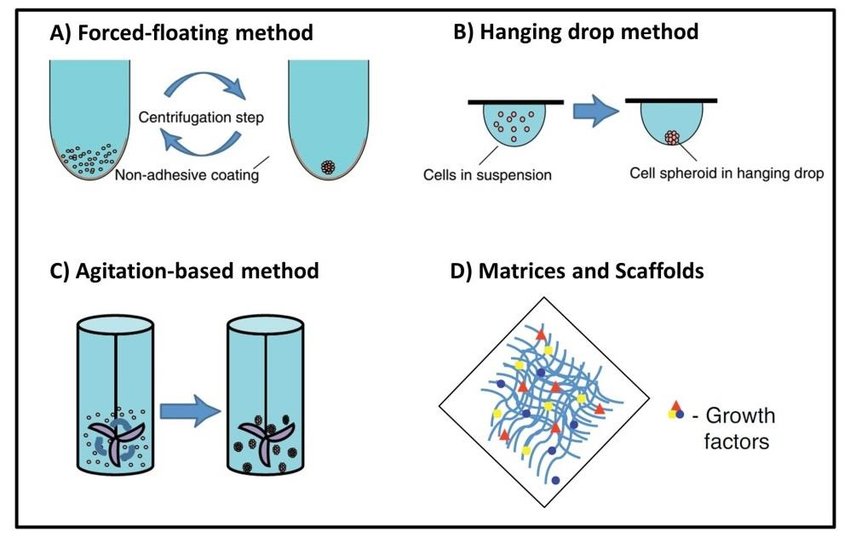
Scaffold free methods to form MCTs
Scaffold free spheroids are formed through agitation dependent techniques, liquid overlay methods, hanging drop cultures or on microfluidic chips. Agitation based techniques involves constant agitation of cells to prevent surface attachment (2,4). Hanging drop method utilizes surface tension and gravity to encourage cell aggregation. Liquid overlay methods involves the use of non-attachment surfaces such as super hydrophobic agar or agarose to promote cell aggregation (2).
These three techniques are economical, and low tech, albeit requiring comprehensive optimization to ensure uniform spheroid formation. Microfluidics is a novel approach and is currently explored widely as it enables the growth of long-term cultures and precise handling of spheroids. Compared to the other scaffold free techniques, microfluidics offer large-scale spheroid generation in precisely controlled conditions making it a feasible option for high throughput drug screens (2,5,6).
Choosing a suitable culture method
The choice of 3D culture to generate MCTs has a significant impact on its reproducibility and reliability. For instance, MCF-7 and MDA-MB-231 cell lines cultivated using agitation based techniques and the hanging drop method produced larger spheroids when compared with those cultures using the liquid overlay technique. Furthermore the former technique also indicated increased collagen type 1 levels than cells cultured using the liquid overlay method (7).
MCT generation was also found dependent upon the type of medium additives used (8). Bladder cancer cell lines such as RT4 was found to form compact spheroids in both hanging drop and liquid overlay methods albeit the growth rate of spheroids was much more efficient in liquid overlay techniques. An assessment of all techniques and the different parameters points towards hanging drop as the more suitable method for forming highly compact and uniform tumor spheroids in most cancer cell lines. However, these techniques must be subjected to further optimization where a standardized and reproducible protocol can be developed to establish formation of MCT spheroids uniform shape and size (3).
References
1. Benien P, Swami A. 3D tumor models: History, advances and future perspectives. Future Oncol. 2014;10(7):1311–27. 43.
2. Nunes AS, Barros AS, Costa EC, Moreira AF, Correia IJ. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol Bioeng. 2019;116(1):206–26.
3. Han, S.J., Kwon, S. & Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int 21, 152 (2021). https://doi.org/10.1186/s12935-021-01853-8
4. Cui X, Hartanto Y, Zhang H. Advances in multicellular spheroids formation. J R Soc Interface. 2017;14:127.
5. Uhl CG, Liu Y. Microfuidic device for expedited tumor growth towards drug evaluation. Lab Chip. 2019;19(8):1458–70.
6. Kwak B, Lee Y, Lee J, Lee S, Lim J. Mass fabrication of uniform sized 3D tumor spheroid using high-throughput microfuidic system. J Control Release. 2018;275:201–7.
7. Raghavan S, Mehta P, Horst EN, Ward MR, Rowley KR, Mehta G. Comparative analysis of tumor spheroid generation techniques for diferential in vitro drug toxicity. Oncotarget. 2016;7(13):16948–61.
8. Froehlich K, Haeger JD, Heger J, Pastuschek J, Photini SM, Yan Y, et al. Generation of multicellular breast cancer tumor spheroids: Comparison of diferent protocols. J Mammary Gland Biol Neoplasia. 2016;21(3–4):89–98.
9. Kuen, Janina. Influence of 3D tumor cell/fibroblast co-culture on monocyte differentiation and tumor progression in pancreatic cancer (2017).