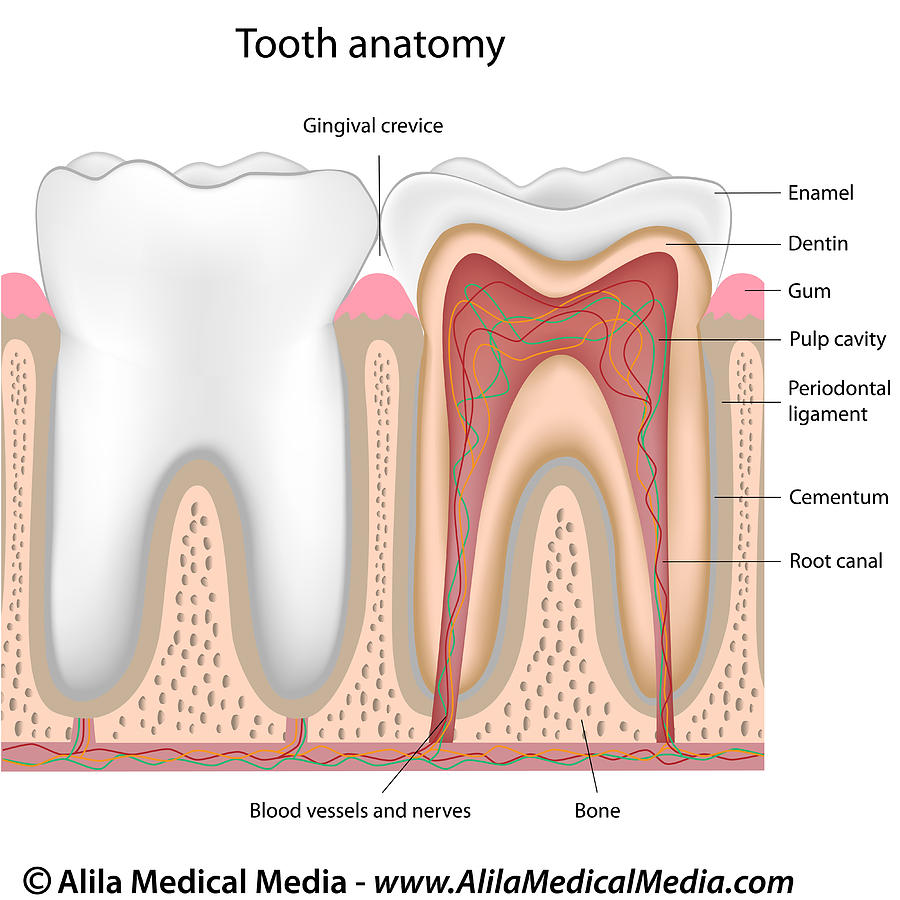
Dental cells
Dental pulp stem cells (DPSCs) are derived from the dental pulp; the soft living tissue within teeth. These cells are pluripotent and are able to differentiate into tissues that bare similarity to mesoderm, endoderm and ectoderm layers (Atari et al, 2012). Additionally they are also able to differentiate in to adipocytes and neural like cells (Gronthos et al, 2002). In the lab, these cells are commonly extracted from postnatal teeth, wisdom teeth and deciduous teeth, thus serving as an efficient and a non-invasive method of extracting stem cells. Consequently, DPSCs serve as a promising source of cells, for tissue engineering applications (Morsczeck and Reichert. 2018).
Limitations of monolayer cultures of DPSCs
Majority of our understanding of the physiological mechanisms involved in dental pulp stem cells rely on data collected from 2D monolayer cell cultures (Baker and Chen, 2012). Recent studies suggests that 2D cells cultures of Dental pulp stem cells, show a more simplified morphology and properties different to the native state. 2D cultures of these stem cells are also unable to replicate the intracellular interactions and cell-extra cellular matrix (ECM) interactions that are necessary to mimic in-vivo like structure and function (Zhang et al, 2018). To this end, 3D cell cultures are an excellent alternative approach as it overcomes the imitations present in 2D cultures and mimic in-vivo conditions.
Scaffold free spheroid cultures
The commonly used 3D culture techniques include, hanging drop method, suspension culture, and scaffold based and scaffold free models, and magnetic levitation (Bu NU et al, 2020) . Among the scaffold free models, the use of ultra low attachment plates coated with specialized polymer solution is excellent for culturing dental pulp stem cells, with in-vivo like structure and function, and increased cell viability. These DPSC spheroids were formed within 24 hours, with increased pluripotent markers, and adipogenic and osteogenic potential, compared to traditional 2D cultures (Xiao and Tsutsui, 2013). In this regard, spheroid cultures of dental pulp stem cells prove to be a rapid, reliable, and physiologically relevant cell culture system for tissue engineering applications.
References
1. Atari, M.; Gil-Recio, C.; Fabregat, M.; García-Fernández, D.; Barajas, M.; Carrasco, M. A.; Jung, H. S.; Alfaro, F. H.; Casals, N.; Prosper, F.; Ferrés-Padró, E.; Giner, L. (2012). “Dental pulp of the third molar: A new source of pluripotent-like stem cells”. Journal of Cell Science. 125 (Pt 14): 3343–56. doi:10.1242/jcs.096537. PMID 22467856.
2. Gronthos, S.; Brahim, J.; Li, W.; Fisher, L. W.; Cherman, N.; Boyde, A.; Denbesten, P.; Robey, P. G.; Shi, S. (2002). “Stem cell properties of human dental pulp stem cells”. Journal of Dental Research. 81 (8): 531–5. doi:10.1177/154405910208100806. PMID 12147742. S2CID 8921170.
3. Morsczeck, C.; Reichert, T. E. (2018). “Dental stem cells in tooth regeneration and repair in the future”. Expert Opinion on Biological Therapy. 18 (2): 187–196. doi:10.1080/14712598.2018.1402004. PMID 29110535. S2CID 41147569.
4. Baker B.M., Chen C.S. Deconstructing the third dimension–how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509.
5. Zhang S., Buttler-Buecher P., Denecke B., Arana-Chavez V.E., Apel C.A. Comprehensive analysis of human dental pulp cell spheroids in a three-dimensional pellet culture system. Arch. Oral Biol. 2018;91:1–8. doi: 10.1016/j.archoralbio.2018.02.008.
6. Bu NU, Lee HS, Lee BN, et al. In Vitro Characterization of Dental Pulp Stem Cells Cultured in Two Microsphere-Forming Culture Plates. J Clin Med. 2020;9(1):242. Published 2020 Jan 16. doi:10.3390/jcm9010242
7. Xiao L, Tsutsui T. Characterization of human dental pulp cells-derived spheroids in serum-free medium: stem cells in the core. J Cell Biochem. 2013 Nov;114(11):2624-36. doi: 10.1002/jcb.24610. PMID: 23794488.