Characterization Of Multicellular Tumor Spheroids
Multicellular tumor (MCT) spheroids are an essential tool in tumor related research, and drug screens. MCT can be defined as aggregates of heterogeneous cells, closely packed with highly dense spheroids. These cells exhibit strong cell-cell and cell-extracellular matrix (ECM) interactions representative of in vivo tumor physiology (1-2). However it if of importance to monitor the development of MCTs in order to ensure the generation of reliable and reproducible cultures that can be used for further study, and high throughput screening.
Structural and morphological monitoring
Typically the development of MCTs is monitored using optical microscopy. Periodic imaging of MCTs allows the study of spheroid volume growth kinetics. Additionally it also yields morphologic information about the MCT to determine its viability and reliability (3).
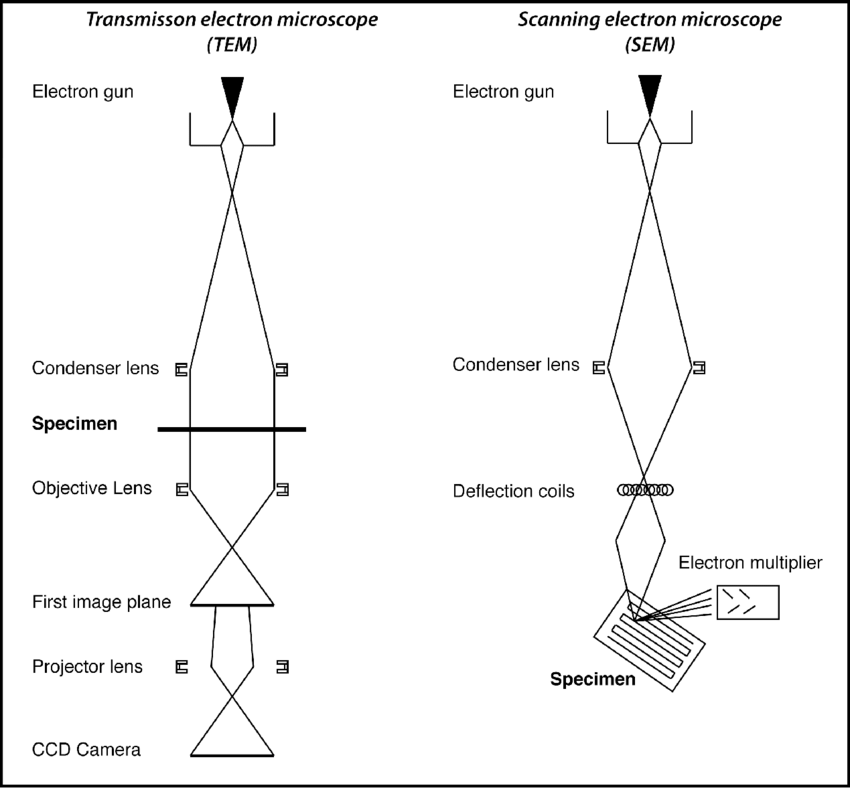
Electron microscopy imaging methods The scanning electron microscope (SEM) is widely utilized as a more reliable method for precise imaging of MCTs, compared with traditional optical microscopy. It is currently used to determine material surface in micrometer and nanometer resolution. For this particular manner of imaging, MCTs are fixed, dehydrated and then coated with conduction materials such as gold-palladium, to obtain precise morphological information of the MCTs (3).
The transmission electron microscope (TEM) is another method to commonly utilized to characterize the internal structure of MCTs. To this end, MCTs are fixed, dehydrated and then sectioned in to thin slices prior to being coated with conduction material. TEM imaging information has been vital especially in determining the drug delivery process of MCTs, and has aided in visualization of drug delivery mechanisms of anticancer therapeutics such as doxorubicin, quantum dots and micelles and their internalization into cells (3-6). Such nano-scale details obtained used these microscopy methods are important in the standardization process to determine the viability, reliability and reproducibility of MCTs in tumor pathophysiological research, as well drug response screens.
References
1. Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–45. 2.
2. Maddaly R, Paramesh V, Kaviya SR, Anuradha E, Paul Solomon FD. 3D cell culture systems: advantages and applications. J Cell Physiol. 2015;230:16–26.
3. Dubois C, Dufour R, Daumar P, Aubel C, Szczepaniak C, Christelle B, et al. Development and cytotoxic response of two proliferative MDAMB-231 and non-proliferative SUM1315 three-dimensional cell culture models of triple-negative basal-like breast cancer cell lines. Oncotarget. 2017;8(56):95316–31.
4. Yuuki S, Norihiko S, Masaki M, Fumio H, Yoko M, Tomio A, et al. Enhanced morphological and functional diferences of pancreatic cancer with epithelial or mesenchymal characteristics in 3D culture. Sci Rep. 2019;9:10871.
5. Hongxu L, Martina HS. Multicellular tumor spheroids (MCTS) as a 3D in vitro evaluation tool of nanoparticles. Small. 2018;14:1702858.
6. Hui-li M, Qiao J, Siyuan H, Yan W, Jin Cui T, Dongliang W, et al. Multicellular tumor spheroids as an in vivo–like tumor model for threedimensional imaging of chemotherapeutic and nano material cellular penetration. Mol Imaging. 2012;11(6):487–98.
7. Hagendorfer, Harald. (2011). New Analytical Methods for Size Fractionated, Quantitative, and Element Specific Analysis of Metallic Engineered Nanoparticles in Aerosols and Dispersions. 10.
