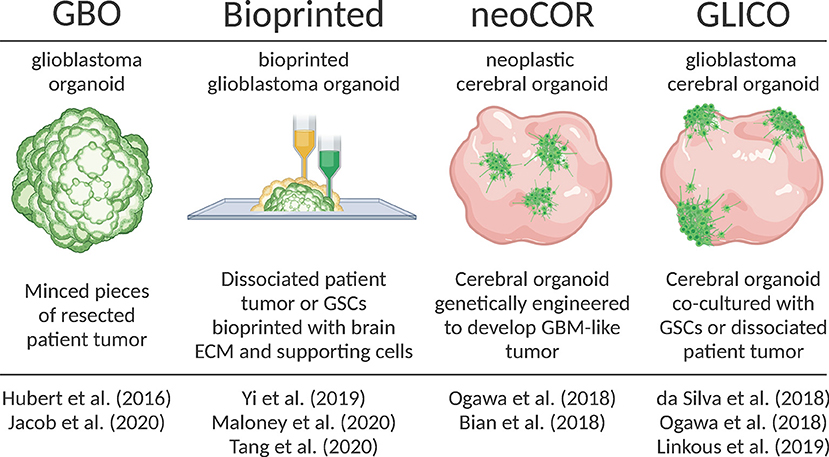
Generate Glioblastoma Organoids
Hubert et al. were the first to report culturing glioblastoma as a more complex organoid composed of multiple cell types rather than relatively homogenous 3D spheroid. They had successfully created these GBM organoids from patient derived primary cultures, xenografts and genetically engineered glioblastomas using Matrigel-based 3D culture methods.
They also created GBM organoids directly from patients’ sample by finely mincing and encapsulating GBM tissues in Matrigel for extended periods of time in vitro. They observed that GBM organoids recapitulate GBM features including cellular morphology and spatial distribution, hypoxic gradient and resistant to radiations (1).
Subsequently, Jacob and team improved upon this model and coined the term GBOs to describe these glioblastoma organoids. They radically reduced the time needed to establish GBOs, further characterized their histological features, cellular diversity, transcriptional profiles and developed a protocol for cryopreserving GBOs. GBOs remained remarkably similar to the patient samples they were derived from.
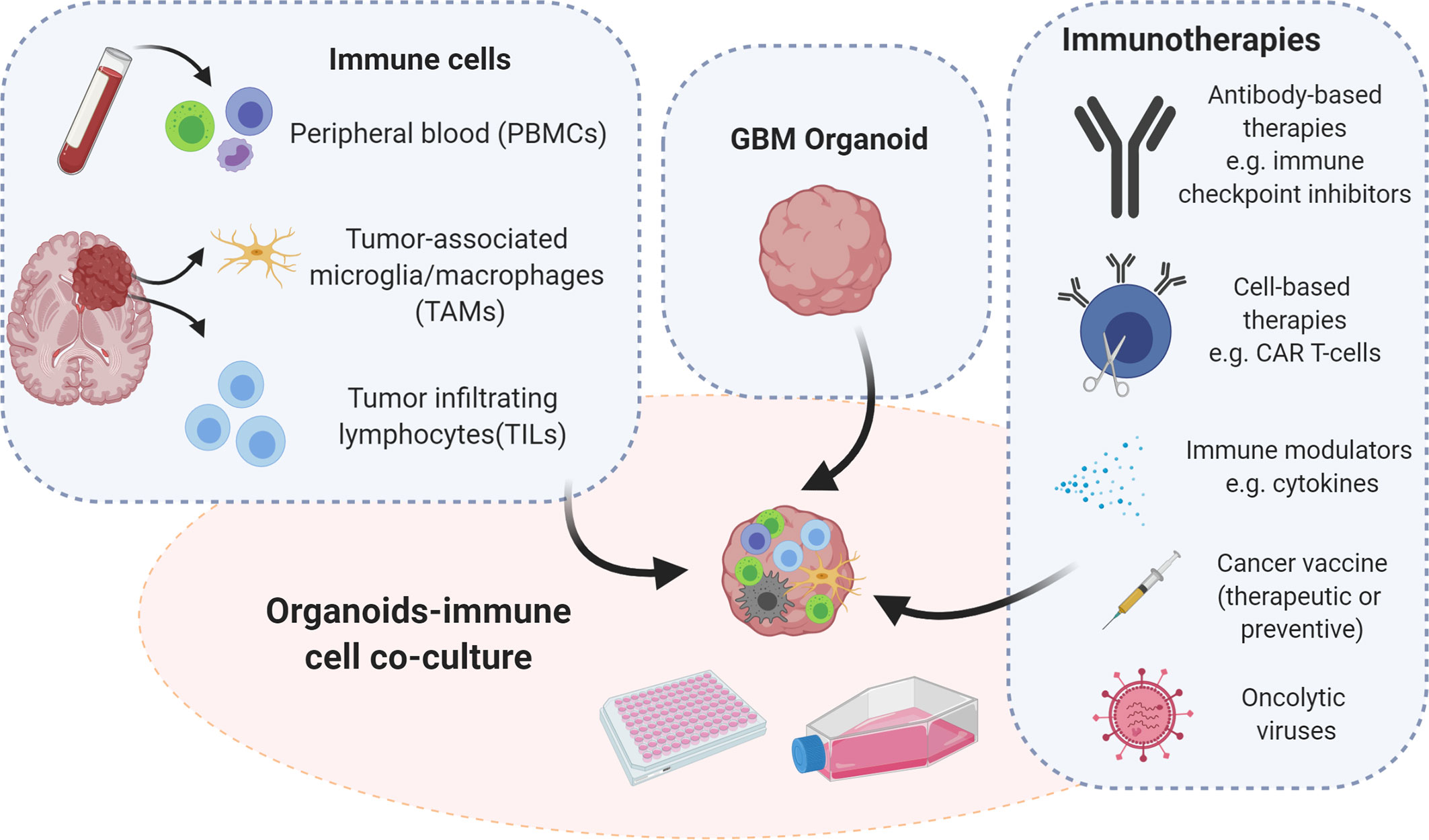
They also showed that GBOs recapitulate patient specific responses to treatment by exposing GBOs to similar post-operative treatment as patient. They also showed that chimeric T cell immunotherapy was most efficient for patient samples with a deletion variant in epidermal growth factors receptor. These results also supported the recent work by Goff et al. and provides support for use of GBOs in personalized medicine. Thus, the short timeframe needed to establish GBOs and to test personalized therapies is a major strength of this model. However few weaknesses includes a lack of normal brain tissue microenvironment, lack of vasculature and limited immune cells (2,3,4).
REFERENCES:
1. Hubert et al., 2016
2. Jacob et al., 2020
3. O’Rourke et al., 2017
4. Goff et al., 2019