96 WELL PLATES AND DEVELOPMENT OF 3D CULTURES: A COMPARISON OF DIFFERENT SPHEOIDS FORMATION METHODS
Cells grown in 3D systems develop into physiologically relevant structures comprising of in-vivo like cellular microenvironment that determine gene expression, growth behavior and specific biochemical functions of organs (1). 3D spheroids of fish and fish cell lines have shown a higher degree and condition of differentiation and metabolic capacity than their monolayer cultures in 2D. Metabolically realistic fish cells, such as those grown in 3D cultures may allow the development of new methods of ecotoxicology which is study of the effects of toxic chemicals on biological organisms. (2)
3D CELL CULTURES AND 96 WELL PLATES
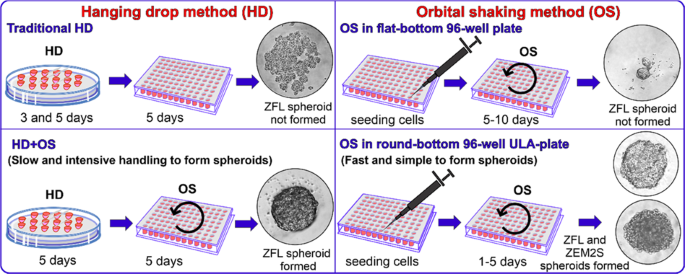
To use spheroid cultures of zebrafish in an ecotoxicity testing, standardization of spheroids cultures must be established. The comparison between the two spheroid culture methods – hanging drop method (3) and orbital shaking method using the cell lines ZFL and ZEM2S from zebrafish. To prevent the attachment of cell aggregates in well plates, wells should be pre-coated with agarose or can also be effectively coated with the Biofloat flex coating solution.
TRADITIONAL METHODS TO DEVELOP 3D CULTURES
Traditional hanging drop method (HD) and hanging drop combined with orbital shaking (OS) (HD+OS) are methods developed based on the spheroid formation protocol of Foty (4). The whole process of spheroid formation must be carried out in a sterile culture condition i.e., under laminar airflow. The basic principle of HD method is the natural disposition of cell aggregate under gravity on the menisci of the hanging droplets of medium without any need for polymer scaffolds, such as Matrigel. This method has been used widely to generate single spheroids of mammalian cells in drops (3). In addition to HD methods, studies shows that gyratory-mediated methods may play a role in the prevention of cellular adhesion on well bottom. The gyratory motion of the culture medium may reduce the affinity of cells to settle at the bottom of the wells, preventing cell attachment onto these plates (1).
All these methods mentioned, have yielded promising results that can are useful for target identification, validation, and toxicity screens as well as pathophysiological studies. With more advances in spheroid culture technology bound to come in future, spheroids offer a rapid, reliable and reproducible 3D culture system.
REFERENCES
1. Baron MG, Mintram KS, Owen SF, Hetheridge MJ, Moody AJ, Purcell WM, Jackson SK, Jha AN (2017). Pharmaceutical metabolism in fish: Using a 3-D Hepatic in Vitro model to assess clearance. PLoS ONE.
2. Hultman MT, Løken KB, Grung M, Reid MJ, Lillicrap A (2019) Performance of three‐dimensional rainbow trout (Oncorhynchus mykiss) hepatocyte spheroids for evaluating biotransformation of pyrene. Environ Toxicol Chem 38:1738–1747
3. Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK (2003). Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Bio- technol. Bioeng
4. Foty R (2011). A simple hanging drop cell culture protocol for generation of 3D spheroids.