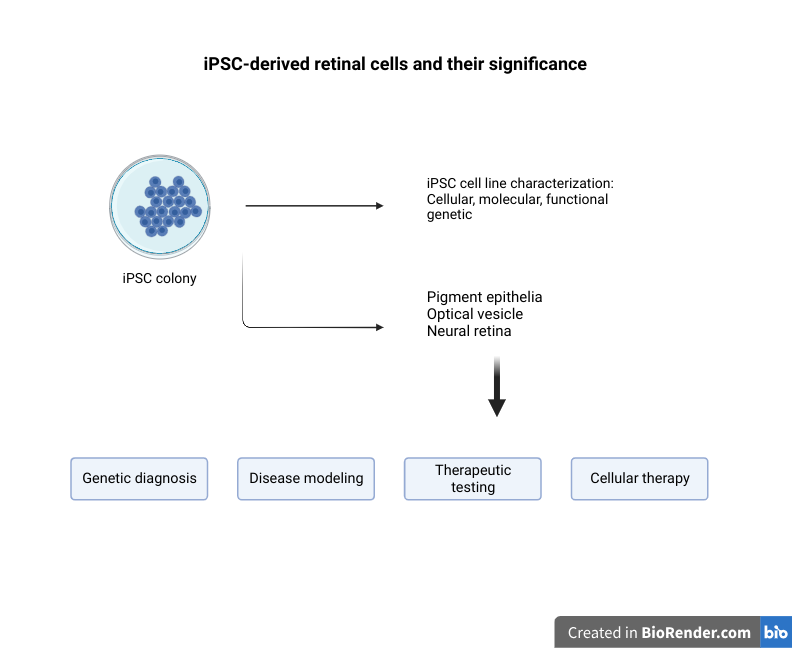
3D Spheroid culture of Stroma cells
Three-dimensional (3D) cultures are widely accepted as an excellent in-vitro model of high through out drug screening, preclinical cancer research and cancer/stem cell research. 3D spheroid models are morphologically and functionally representative of in-vivo cell state, and are able to reproduce characteristics of solid tumors, unlike the conventional 2D cultures (1).
What are stroma cells?
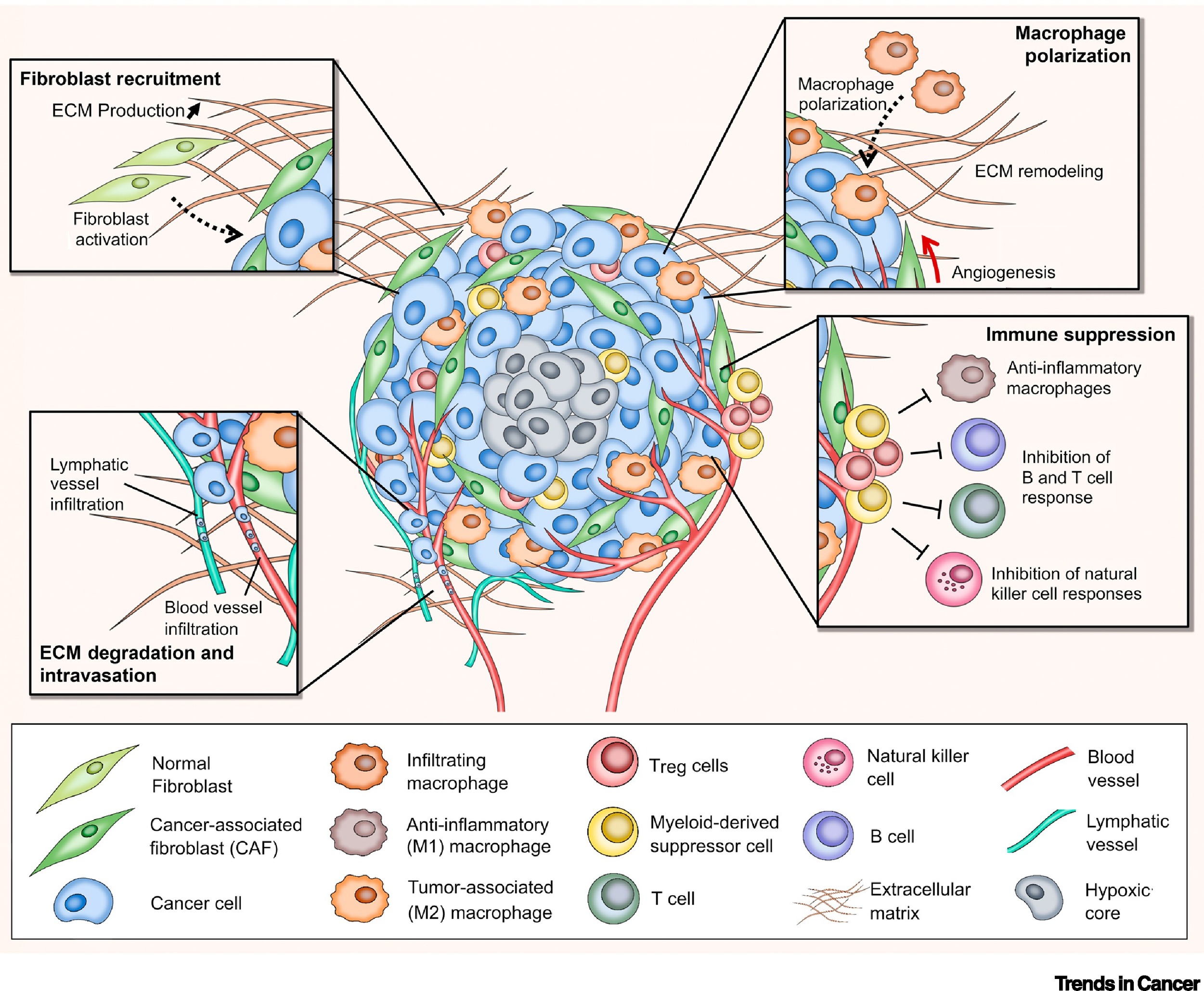
Stroma is the section of a tissue or organ that provides structure or connective support. For e.g. the stroma of an organ consists of connective tissue, blood cells, ducts etc. while the functional role of the organ is played by a group of cells collectively termed as the parenchyma. Stromal cells are primarily abundant in the bone marrow but are also present all around the body. The most common stromal cells are fibroblasts and pericytes. In addition to providing support to the parenchyma, stromal cells modulate the immune system and inflammation through multiple pathways (2). They help in hematopoietic cell differentiation and formation of the essential blood elements. In a pathological context, stromal cell interactions with tumor cells play an important role in cancer growth and progression (2).
Spheroid culturing of stromal cells
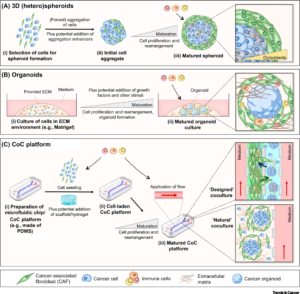
Interaction of tumor cells with stroma plays an important function in tumor growth survival and spread. To this effect, fibroblasts and macrophages are involved in stromal tissue production and maintenance of tumor microenvironment. In the past, most tumor 3D cell culture models were composed of exclusively tumor cells (3,4). The lack of tumor stromal cells and the extra cellular matrix, in these cultures, only partially mimicked the in-vivo cell state. However, recent advances in the spheroid cell culture has allowed for co-culture systems, in which tumor cells are co-cultured with stromal cells such as 3T3 fibroblasts to better represent the tumor microenvironments (4,5). Spheroid co-cultures can be generated using scaffolding or self-assembled aggregates such as spheroids cultured on low attachment plates. These methods are relatively simple, reliable and can achieve high throughput capabilities (4). Co-culturing of tumor spheroids with fibroblasts were found effectively promote proliferation, scattering and tumor cell invasiveness, thus providing an excellent translatable platform for high throughput drug screens, and preclinical cancer studies.
References
1. Shao H, Moller M, Wang D, Ting A, Boulina M, Liu ZJ. A Novel Stromal Fibroblast-Modulated 3D Tumor Spheroid Model for Studying Tumor-Stroma Interaction and Drug Discovery. J Vis Exp. 2020 Feb 28;(156).
2. Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 296 (5570): 1046–9. 2002
3. João Rodrigues, Marcel A. Heinrich, Liliana Moreira Teixeira, Jai Prakash, 3D In Vitro Model (R)evolution: Unveiling Tumor–Stroma Interactions. Trends in Cancer, Volume 7, Issue 3, (2021). Pages 249-264.
4. Rama-Esendagli D, Esendagli G, Yilmaz G, Guc D. Spheroid formation and invasion capacity are differentially influenced by co-cultures of fibroblast and macrophage cells in breast cancer. Mol Biol Rep. 2014 May;41(5):2885-92. 5. https://facellitate.com/wp-content/uploads/Establishment-of-perfect-3D-spheroids-for-1.pdf