3D models of cancer cells
Tumor cells typically grow in 3-dimentional configurations. The surrounding tumor microenvironment is composed of non-tumor cells, and extracellular matrix (ECM). Within this microenvironment, tumor cells face hard growth conditions that include low oxygen exposure and nutrient levels and a susceptible to cell-cell interactions and cellular signals from surrounding non- tumor cells.
In traditional 2D monolayer cultures the surrounding microenvironment is not replicated in-vitro, thus yielding cellular behaviour that is not representative of that in vivo. To this effect, in the recent decades, many 3D cell culture techniques have been developed as an attempt to recreate in-vivo growth conditions of tumor cells, to obtain a more accurate and comprehensive view of tumor cell behaviour (1).
3D culture models of cancer cells : A classification
The presently available 3D models of cancer cells differ in3 ways namely, source of cancer cell, handling protocol, and time taken to produce 3D cultures. There are four main techniques for obtaining a 3D model for cancer cells.
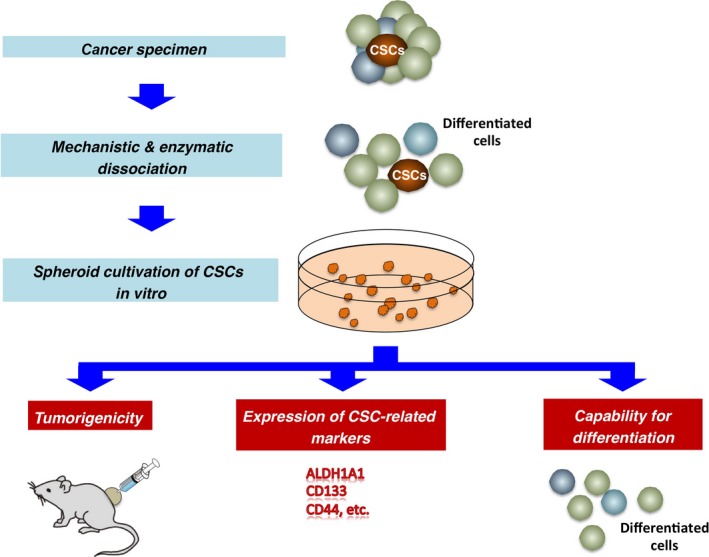
1) Organotypic multicellular spheroids/organotypic explants
– Method in brief: Cancer cells are mechanically dissociated, and cultivated together with the non-tumor cells and stromal cells that are typically found within the tumor microenvironment in vivo.
– Advantages: The cancer cells retain majority of the phenotypic features, and the cellular heterogeneity of the primary cancer.
– Variations: Cancer tissue originated spheroids (cancer tissues are mildly dissociated with enzymatic treatments to extract heterogeneous cancer spheroids) (2).
2) Multicellular tumor spheroids
– Method in brief: Cultures are produced from cancer cell lines, in conventional media supplemented with FBS. Methodologically similar to 2D cultures, with the exception of cells being cultivated in a suspension in spheres to facilitate the cell-cell adhesion.
– Advantages: Albeit with limited histological resemblance, these cultures closely mimic metabolic and proliferative gradients associated with in vivo tumors, and indicate clinically representative, multicellular chemoresistance. The cultures are easily reproduced, low maintenance requirements, with easily introduced genetic manipulations. Prime candidate for high throughput drug screens (3,4).
3) Tumor derived organoids
– Method in brief: Cells are cultivated under precise growth conditions that include a basement membrane matrix/scaffold, Wnt agonists, tyrosine kinase receptor agonists, and bone morphogenetic proteins. Multi step carcinogenesis model can be developed by sequential introductions of tumor suppressor gene mutations in non-tumor organoids.
– Advantages: Presently serves as a powerful platform for ex-vivo organogenesis (5-8).
4) Tumor derived spheroids (tumorospheres)
– Method in brief: Culturing techniques are similar to multicellular tumor spheroids. Additionally serum free culture medium can be employed to produce stem cell like cell states.
– Advantages: Mainly used as a supporting system to analyze cancer stem cell characteristics in- vitro.
References
1) Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017 Mar; 108(3):283-289.
2) Kondo J, Endo H, Okuyama Het al. Retaining cell-cell contact enablespreparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci USA 2011; 108: 6235–40.
3) Durand RE, Olive PL. Resistance of tumor cells to chemo- and radiotherapy modulated by the three-dimensional architecture of solid tumors and spher-oids. Methods Cell Biol 2001;64: 211–33.7
4) Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc 2009;4: 309–24.
5) Sato T, Stange DE, Ferrante Met al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011; 141: 1762–72.9
6) Clevers H. Modeling development and disease with organoids. Cell 2016; 165: 1586–97.10
7) Sato T, Clevers H. SnapShot: growing organoids from stem cells. Cell 2015; 161: 1700.e1.11
8) Drost J, Artegiani B, Clevers H. The generation of organoids for studying Wnt signaling. Methods Mol Biol 2016; 1481: 141–59.
9) Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia 2015; 17:1–15.
