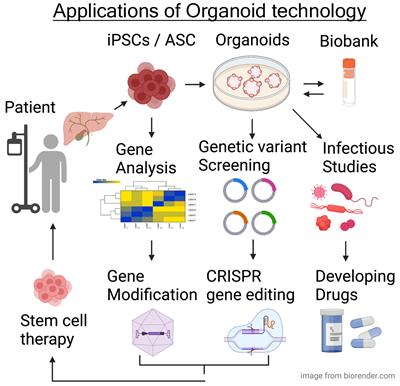
Current limitations of organoid technology
Three-dimensional (3D) organoid technology has become popular among researchers in the past decade because of its ability to recapitulate the physiology and genetics of organs or tissue samples. Despite the promising applications of organoids in basic research, a lot of improvements are required to expand their translational application.
1. Lack of a vascular system
The human body is dynamic; blood constantly flows through the blood vessels to exchange nutrients, oxygen, and waste ensuring cell survival. However, organoids lack a vascular system and are cultured through static methods. As the organoids keep growing, the cells in the center are unable to get enough nutrition and the exchange of waste becomes difficult. Other factors that affect the vascularization of organoids include cytokine concentration, the shape of the organoid, and flow rate.
Currently, there are two main methods that scientists use to generate vascularized organoids: “in vivo vascularization” (1) and “in vitro vascularization” (2). In in vivo vascularization, organoids are transplanted into the corresponding animal model to establish a connection between the host and the transplanted organoid model through blood vessels. In vitro organoid vascularization I carried out by addition of vascular cells or genetic engineering.
2. Organoids and immune cells
The immune system comprises immune organs, immune cells, and regulatory proteins that work together to protect the body from foreign invaders. Epithelial cells and immune cells interact to maintain the physiological balance in humans. It is difficult to establish an interacting and integrating model of organoids and immune cells to study disease models, therapy efficacies, and drug evaluation.
Current culture technologies cannot meet in vivo simultaneous interactions of tumor and immune cells. Tumor organoids with an established immune system will help to study the disease in detail, produce clinically relevant results, and reduce the time between the laboratory and the hospital (3).
3. Organoids culture medium
Matrigel is widely used to cultivate organoids as it provides them with an extracellular matrix enabling the investigation of cell-matrix interactions. Matrigel is derived from the secretion of Engelbreth-Holm-Swarm mouse sarcoma cells and includes laminin, collagen, heparan sulfate proteoglycan, and entactin. Despite these advantages, Matrigel is extremely complex and contains more than 180 unique proteins.
The impossibility of precisely defining its composition makes it difficult to fully understand its influence on organoids’ structure and function. Matrigel may also lack important components that are necessary for the cultivation of organoids in optimal conditions. Further, Matrigel’s animal origin limits its use in human clinical applications. Considering these disadvantages, there is a need to develop Matrigel-independent organoid culture methods (4,5).
References
1. van den Berg, C. W., Ritsma, L., Avramut, M. C., Wiersma, L. E., van den Berg, B. M., Leuning, D. G., et al. (2018). Renal Subcapsular Transplantation of PSC-Derived Kidney Organoids Induces Neo-Vasculogenesis and Significant Glomerular and Tubular Maturation In Vivo. Stem Cell Rep. 10 (3), 751–765. doi:10.1016/j.stemcr.2018.01.041
2. Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 2019 Mar;16(3):255-262. doi: 10.1038/s41592-019-0325-y. Epub 2019 Feb 11. PMID: 30742039; PMCID: PMC6488032.
3. Campinoti S, Gjinovci A, Ragazzini R, Zanieri L, Ariza-McNaughton L, Catucci M, Boeing S, Park JE, Hutchinson JC, Muñoz-Ruiz M, Manti PG, Vozza G, Villa CE, Phylactopoulos DE, Maurer C, Testa G, Stauss HJ, Teichmann SA, Sebire NJ, Hayday AC, Bonnet D, Bonfanti P. Reconstitution of a functional human thymus by postnatal stromal progenitor cells and natural whole-organ scaffolds. Nat Commun. 2020 Dec 11;11(1):6372. doi: 10.1038/s41467-020-20082-7. PMID: 33311516; PMCID: PMC7732825.
4. Andrews MG, Kriegstein AR. Challenges of Organoid Research. Annu Rev Neurosci. 2022 Jul 8;45:23-39. doi: 10.1146/annurev-neuro-111020-090812. Epub 2022 Jan 5. PMID: 34985918.
5. Kozlowski MT, Crook CJ, Ku HT. Towards organoid culture without Matrigel. Commun Biol. 2021 Dec 10;4(1):1387. doi: 10.1038/s42003-021-02910-8. PMID: 34893703; PMCID: PMC8664924